AuPd-nNiO as an effective catalyst for the base-free oxidation of HMF under mild reaction conditions†
Abstract
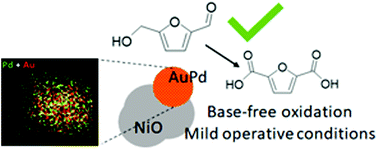
Au-Based catalysts supported on nanosized NiO (nNiO) were synthesized and were investigated in the oxidation of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid (FDCA) under base-free conditions using molecular oxygen as the oxidant, at 90 °C. By choosing the optimal composition of Au–Pd nanoparticles (6 : 4 Au/Pd atomic ratio), we report an efficient and stable nNiO-supported Au–Pd alloy catalyst. The presence of nNiO and Au–Pd nanoparticles on the surface was essential to achieve high conversion (95%) and high activity, high yield of FDCA (70%) and good level of stability. Significant synergistic effects were observed between Au and Pd in the alloy as well as on NiO. The present work provides mechanistic insights into the alloying effect and support-metal interaction in terms of understanding better the role of the alloy and support in affecting specific reaction pathways. Finally, the outcome of this knowledge can help develop efficient catalysts for the aerobic oxidation of biomass-derived molecules under base-free conditions in water and under mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...