Continuous separation of cytochrome-c PEGylated conjugates by fast centrifugal partition chromatography†
Abstract
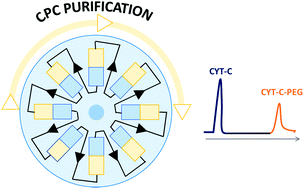
Herein, the effective use of aqueous biphasic systems (ABS) in Fast Centrifugal Partition Chromatography (FCPC) for the purification of PEGylated cytochrome c conjugates is shown. High recoveries (between 88% and 100%) and purities (∼100%) were obtained. Both the unreacted cytochrome c and solvents may be recovered and reused, thus allowing the design of a sustainable process in a continuous regime for the isolation of bioconjugates. This process allowed the reduction of the complete E-factor and carbon footprint at circa 100% and 67%, respectively, reinforcing the important environmental contribution of recycling units.


 Please wait while we load your content...
Please wait while we load your content...