Engineering enzymatic cascades for the efficient biotransformation of eugenol and taxifolin to silybin and isosilybin†
Abstract
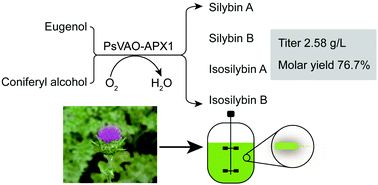
Silybin and isosilybin are the major active components of silymarin, which have long been used to treat liver disease. Market available silymarin is currently extracted from the seed of milk thistle (Silybum marianum Linn. Gaertn) as a mixture, which consists of at least 7 flavonolignans including silybin and isosilybin, and other flavonoids. Plant extraction suffers from limited yield, geographical variations and market dynamics due to insufficient supply and inconsistent product purity. There is a pressing need to develop cost-efficient processes to manufacture silybin-based drugs. Silybin and isosilybin biosynthesis involves the oxidation of the expensive precursor coniferyl alcohol by milk thistle ascorbate peroxidase (APX1). However, the limited supply of coniferyl alcohol restricts the efficient production of silymarin-based drugs. In this study, we seek to develop alternative routes for the synthesis of silybin and isosilybin. Enzymology studies led us to identify that APX1 was not stereoselective and eugenol could be used as a surrogate substrate for silybin production. We then established an enzymatic cascade, which consists of Penicillium simplicissimum vanillyl alcohol oxidase (PsVAO) and APX1, for the efficient conversion of eugenol and taxifolin to silybin and isosilybin. Using H2O2 as the electron acceptor, APX1 converts coniferyl alcohol and taxifolin to silybin, and detoxifies the byproduct H2O2 at the same time. With cofactor heme enhancement, reaction condition optimization and scale-up, our enzymatic cascades yielded 2.58 g L−1 silybin and isosilybin. This efficient enzymatic cascade represents the first route to produce silybin and isosilybin by microorganisms. The coupling of PsVAO with APX1, and the detoxification of H2O2 by APX1 also offers a promising approach to generate silybin-based drugs.


 Please wait while we load your content...
Please wait while we load your content...