Open Access Article
Open Access ArticleSafer bio-based solvents to replace toluene and tetrahydrofuran for the biocatalyzed synthesis of polyesters†
Alessandro
Pellis
 *,
Fergal P.
Byrne
*,
Fergal P.
Byrne
 *,
James
Sherwood
*,
James
Sherwood
 ,
Marco
Vastano
,
Marco
Vastano
 ,
James W.
Comerford
,
James W.
Comerford
 and
Thomas J.
Farmer
and
Thomas J.
Farmer

University of York, Department of Chemistry, Green Chemistry Centre of Excellence, Heslington, York, YO10 5DD, UK. E-mail: ale.pellis@york.ac.uk; alessandro.pellis@gmail.com; fergal.byrne@york.ac.uk
First published on 7th March 2019
Abstract
With increased awareness of environmental issues caused by traditional petrochemical processes, both academia and industry are making enormous efforts towards the development of sustainable practices using renewable biomass as a feedstock. In this work, the biocatalyzed synthesis of polyesters derived from renewable monomers was performed in safer, bio-derivable organic solvents. Candida antarctica lipase B (CaLB), an enzyme belonging to the Ser-hydrolase family (adsorbed on methacrylic resin, also known as Novozym 435) was tested for its performance in the synthesis of adipate- and furandicarboxylate-based polyesters. In addition, the traditional solvents toluene and tetrahydrofuran were compared with a series of green solvents, 2,2,5,5-tetramethyloxolane, 2-methyltetrahydrofuran, 2,5-dimethyltetrahydrofuran and pinacolone for the enzymatic polymerizations. We can conclude that the monomer conversions and molecular masses of the obtained polyesters in all the tested alternative solvents were suitable, and in some cases superior, with CaLB immobilized via physisorption on acrylic resin being the optimal biocatalyst for all reactions. Strikingly, it was found that for the majority of the new solvents, lower reaction temperatures gave comparable monomer conversions and polymers with similar molecular weights whilst pinacolone yielded better polymers with Mn > 2000 Da and conversions of over 80%.
Introduction
Society's concern towards the use of toxic catalysts and solvents in industrially-relevant manufacturing processes, ranging from synthetic chemistry to the coating industry, is driving the development of new sustainable processes that have a lower impact on the surrounding environment and to public health. For example, many volatile, non-polar hydrocarbons, such as hexane, benzene and toluene, have been identified as hazardous air pollutants, carcinogens, mutagens and reprotoxins.1–4Examples of green alternatives to traditional low polarity solvents, such as toluene and hexane, have been presented in recent years. Terpenes and their derivatives have been demonstrated as valuable bio-based hydrocarbon solvents (e.g. limonene and para-cymene) but despite having similar solubility properties, they possess higher boiling points (>170 °C) rendering their removal difficult and energy intensive, and furthermore exhibit aquatic toxicity. 2-Methyltetrahydrofuran (2-MeTHF) is a volatile ether which can be produced by the chemocatalytic treatment of biomass, and its use has been demonstrated in several applications.5–7 Now regularly used in chemical process development, 2-MeTHF is arguably the most successful neoteric bio-based solvent. More recently, a bio-derivable alternative to toluene has been described. 2,2,5,5-Tetramethyloxolane (TMO) possesses a comparable boiling point (112 °C) to toluene (111 °C) and although it is an ether, it does not form hazardous peroxides.8 Several esters and ketones have also recently been suggested as alternatives to toluene, displaying a similar solubility profile and performance in chemical reactions.9
Along with the need for safer organic media, there has been great interest in the development of sustainable, non-toxic catalysts, particularly in the form of low cost biocatalysts, that can be used as catalysts in non-aqueous environments.10 Lipases, in their immobilized form, have emerged as key biocatalysts for the synthesis of short chain esters, chiral pharmaceutical compounds and polymers such as polyesters and polyamides.11–13 In particular, Candida antarctica lipase B (CaLB) physically adsorbed on polymethylacrylate beads (also known as Novozym 435) resulted in a highly active and stable biocatalyst in organic media.14,15 Further to this, Novozym 435 has been shown to be active when used in conjunction with various green solvents such as the synthesis of fatty esters,16 and the esterification of 2-phenylpropionic acid in a flow system.17
In the present work, a series of enzymatically-synthesized furan-functionalized bio-based polyesters were prepared via polycondensation in a selection of neoteric solvents; 2-methyltetrahydrofuran (2-MeTHF), 2,5-dimethyltetrahydrofuran (DMeTHF), TMO and pinacolone. Traditional solvents toluene and tetrahydrofuran (THF) were used as a comparison.14 In addition, several immobilized formulations of Candida antarctica lipase B (covalently immobilized or adsorbed) and a cutinase from Aspergillus (Novozym® 51032) immobilized via adsorption were tested as biocatalysts. The collected data allowed the interactions between solvent, monomer and enzyme to be examined, and it was found that the new generation of solvents produced superior polymers (higher monomers conversion and molecular masses) at a lower reaction temperature than the traditional solvents or with no solvent.
Results and discussion
Enzymatic synthesis of aliphatic polyesters
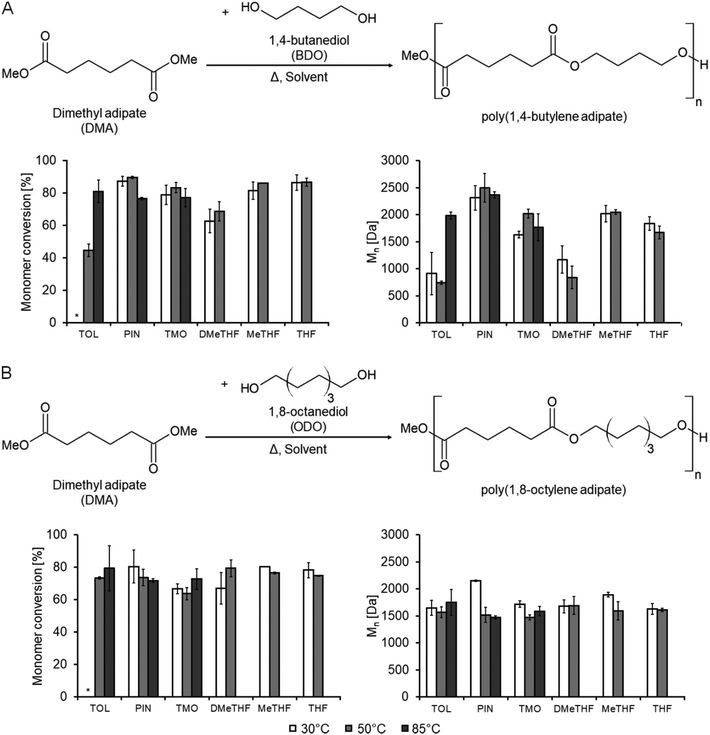
The bio-based production of adipic acid and 1,4-butanediol (BDO) is currently performed on an industrial scale using fermentative methods.18 The biocatalyzed polycondensation process is usually conducted in solventless conditions, as the use of a solvent in otherwise identical reaction conditions results in similar monomer conversions and obtained molecular masses but lower dispersity (Đ). As such, this was chosen as a model reaction for the present work to investigate unconventional solvents and biocatalytic effects.The solvents were chosen so that they would dissolve the monomers and the resulting polymer (except for toluene at 50 °C where BDO is initially only partially soluble) while allowing the enzyme to remain active. Biocatalysts lose most of their activity in extremely polar solvents (due to water stripping) and in solvents containing halogen atoms such as chloroform.16,17 In addition, it was required that the solvent could be easily removed after the reaction, so they needed to be volatile, preferentially in the ideal 70–139 °C range, as set by the CHEM21 solvent selection guide.19 Boiling points higher than this makes recovery by distillation increasingly energy intensive; any lower and fugitive emissions can cause air pollution. All the solvents (except THF) used in this study were within this range. Esters and protic solvents were not used due to the likelihood of side-reactions. These requirements considered, four green solvents were suitable for investigation; pinacolone, TMO, DMeTHF and 2-MeTHF. Toluene has similar dipolarity to the green solvents, but lacks the coordinating oxygen of the oxolane family of solvents. As shown in Fig. 1, all the tested solvents were found to be suitable media for the reaction since poly(1,4-butylene adipate) was obtained in all cases but with different monomer conversion rates.
The reaction was initially conducted at 85 °C, which was reported as the optimal temperature for the polymerization of the same monomers in solventless bulk reaction systems (Fig. 1A and B).20 1,8-Octanediol (ODO) was also investigated as an alternative to BDO for comparison. Toluene, TMO and pinacolone were used as the initial reaction media. No significant differences in terms of monomer conversions were observed at 85 °C in either the BDO or ODO systems. Molecular masses were also similar, although pinacolone produced a polymer with a particularly high molecular mass in the BDO system.
The temperature of both reactions was reduced to 30 and 50 °C to accommodate the lower boiling solvents (2-MeTHF, DMeTHF and THF). In the case of the ODO reaction, there was very little difference in performance between solvents in terms of monomer conversion and polymer molecular mass except in the case of pinacolone, which at 30 °C achieved excellent conversions (80%) and a Mn > 2000 Da, showing a significant advantage in terms of productivity and lower energy consumption. The indifference to the choice of solvent permits the selection of an environmentally friendly option instead of the petrol-based toluene or THF.
Conversely, the reaction of BDO at 50 °C illustrated clear differences between each solvent. It can be seen that both conversions and molecular masses increased using pinacolone despite the lower temperature, whereas the reaction in toluene was suppressed (monomers conversions of 45% vs. >83% and Mn of 744 Da vs. >2000 Da). The monomer conversion also appeared to increase across the series of ethers DMeTHF and 2-MeTHF vs. THF, with the obtained Mn following a similar trend.
It is impressive that the reaction performed in pinacolone at 30 and 50 °C was superior to the previously reported solventless process; the polycondensation in pinacolone gave monomer conversions of 90% and Mn of 2500 Da in comparison to a similar conversion of 89% but a slightly lower Mn (2200 Da) obtained in the solventless bulk reaction system at higher operational temperature (85 °C).20 Moreover, the Đ of the polymers synthesized in pinacolone (Đ = 1.20) is also slightly lower than those produced in the melt (Đ = 1.31) therefore constituting another advantage of using pinacolone as a component of the reaction mixture (for the complete set of data please see ESI, Tables S1 and S2†).
The reaction was also carried out at 30 °C to reduce the energy demand of the process further. A similar trend was observed at this temperature as at 50 °C, but it was found that toluene was unable to dissolve either diol at this temperature, leading to rather low Mn (<1000 Da).
Overall, the green aspects of the new process described in this work are excellent. While an organic solvent has been utilized, a green solvent has been demonstrated to be better than two traditional solvents, toluene and THF. In addition, the monomer conversions and molecular mass of the polymers have been improved, resulting in a superior quality polymer, with the reaction temperature being significantly reduced.
Enzymatic synthesis of poly(1,4-butylene adipate) using various enzymatic preparations
The biocatalyzed synthesis of poly(1,4-butylene adipate) was also carried out using other CaLB formulations (immobilized in different ways) and a commercial cutinase from Aspergillus belonging to the same Serin-hydrolase family (Table 1). Changing the enzymatic preparation led to different conversions and molecular masses but the trend observed at the different reaction temperatures and by using the various solvents was fully preserved.| Enzyme | Immobilization method | Catalog code | Activity [PLU g−1] | Vendor |
|---|---|---|---|---|
| Candida antarctica lipase B | Covalently immobilized | IMMCALB-T2-150 | 5000 | Chiralvision |
| Cutinase from Aspergillus (NZ 51032, cutinase1) | Adsorbed on acrylic resin | IMML51-T1-350 | 3000 | |
| Recombinant Candida antarctica lipase B | Adsorbed on Purolite's highly hydrophobic carrier ECR1030M | — | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
c-Lecta |
The best results in terms of conversions and molecular masses were obtained with Novozym 435, the CaLB formulation used throughout this work. The 2nd best performing preparation was the adsorbed CaLB from c-Lecta which was also the enzyme found to be the most stable at the operational conditions (magnetic stirring at 400 rpm). While all the other preparations were quickly ground into powders within 15 min of reaction, the c-Lecta preparation was relatively stable (minor visible sediment) for up to 2 h, at which point it started to be destroyed by the vigorous agitation used.
The CaLB from Chiralvision ranked 3rd while the cutinase from Aspergillus purchased from the same company was the least active enzyme for the above-mentioned reaction (see ESI, Fig. S1–S12† for the complete set of data).
The recycling of the biocatalyst was not in the scope of the present manuscript since alternative reaction systems that allow the preservation of the biocatalyst's integrity and its reuse for over 8 cycles have already been reported.21,22
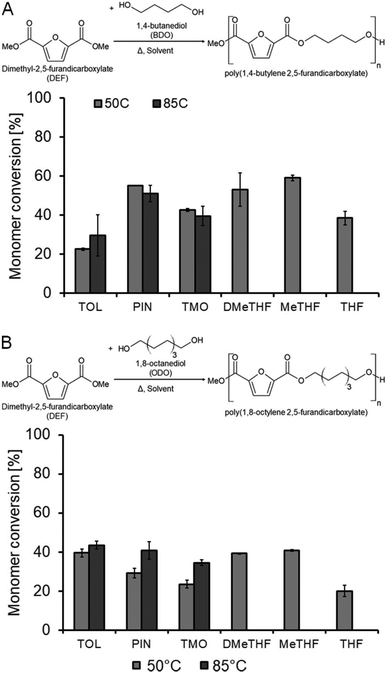
Enzymatic synthesis of furan-based polyesters
Due to the increasing interest in the synthesis of furan-derived plastic alternatives due to their bio-based nature, our investigation was extended to the synthesis of poly(1,4-butylene-2,5-furandicarboxylate) and poly(1,8-octylene-2,5-furandicarboxylate). Again, the suitability of the various solvents for such syntheses was evaluated and the results can be seen in Fig. 2. For the reactions of diethyl-2,5-furandicarboxylate, GPC analysis gave no conclusive results since the obtained oligomers had very limited molecular weights that were below the calibration range (used standards: Sigma-Aldrich polystyrene standards, range: Mp = 400–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da).
000 Da).
Monomer conversions were lower in the polymerization of both BDO and ODO with diethyl-2,5-furandicarboxylate compared to with dimethyl adipate. The BDO system at 50 °C showed toluene and THF to be the worst performing solvents (Fig. 2A). Interestingly, no improvement in conversion was obtained using toluene when the reaction temperature was increased to 85 °C, unlike in the adipate system. When ODO was used in 50 °C reactions, toluene, DMeTHF and 2-MeTHF were all found to obtain the best monomer conversion (39–41%), followed by pinacolone and TMO, with THF being the worst solvent (29, 24 and 20% respectively). Swelling of the resin support was suspected of being the cause of the differences in conversion, but an investigation into this did not produce a valid relationship between solvent polarity and swelling.
Thermal analysis of the synthesized polyesters
To fully characterize the polyesters synthesized in the different conditions (by varying the solvent and operational temperature), we also performed thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) to characterize the obtained reaction products.From Fig. 3 it is possible to observe the thermal degradation profiles of poly(1,4-butylene adipate) (PBA) synthesized in different solvents and at different temperatures. The onset temperature of degradation (Td) is related to the molecular weight of the obtained oligomers. The PBA synthesized in toluene at 50 °C has a degradation temperature significantly lower than the samples synthesized in TMO and pinacolone (T10% = 193 °C for toluene vs. 346 °C and 357 °C for TMO and pinacolone respectively, Fig. 3A). For the reactions conducted in the three above mentioned solvents at 85 °C, with the molecular weights rather similar, no major differences in the degradation temperatures were observed, with the synthesized PBA having a T10% of 342–353 °C (Fig. 3B). In Fig. 3C, the decomposition temperature of PBA synthesized in THF, MeTHF and DMeTHF at 30 °C is shown. As expected, the thermal degradation profiles represent the polymer's molecular mass as shown in Fig. 1A. The complete set of TGA data reporting the temperature at which degradation reaches 5%, 10% and 50% for the samples plotted in Fig. 3 is presented in Table 2.
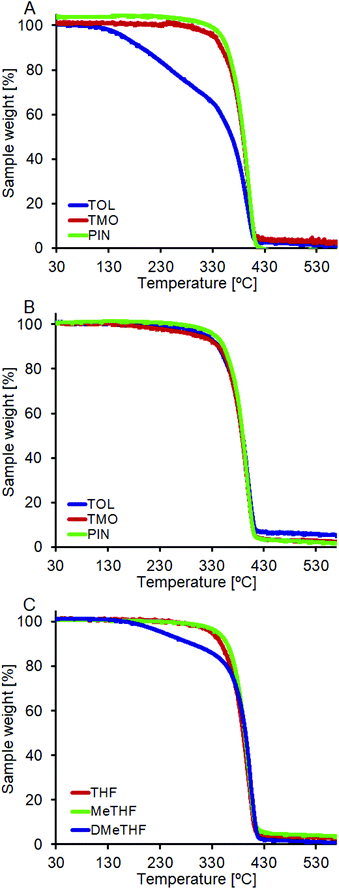 | ||
| Fig. 3 TGA of PBA synthesized in (A) TOL, TMO and PIN at 50 °C; (B) TOL, TMO and PIN at 85 °C and (C) THF, MeTHF and DMeTHF at 30 °C. | ||
| Solvent | Reaction T [°C] | T 5 [°C] | T 10 [°C] | T 50 [°C] |
|---|---|---|---|---|
| TOL | 50 | 159 | 193 | 367 |
| TMO | 335 | 346 | 388 | |
| PIN | 347 | 357 | 389 | |
| TOL | 85 | 317 | 343 | 389 |
| TMO | 301 | 342 | 387 | |
| PIN | 336 | 353 | 389 | |
| THF | 30 | 329 | 347 | 390 |
| MeTHF | 342 | 359 | 393 | |
| DMeTHF | 237 | 292 | 395 |
The DSC analysis of selected samples was also performed and clearly shows that all synthesized polymers: poly(1,4-butylene adipate), poly(1,8-octylene adipate), poly(1,4-butylene 2,5-furandicarboxylate) and poly(1,8-octylene 2,5-furandicarboxylate), have crystalline characteristics. The DSC data also show a clear difference in the melting (Tm) and crystallization (Tc) temperatures of the polymers, again related to their molecular weight. Oligomers having higher molecular masses melt and crystallize at higher temperatures compared to the oligomers synthesized at the same operational temperature (Table 3).
| Solvent | Reaction T [°C] | T c [°C] | First Tm [°C] | Second Tm [°C] |
|---|---|---|---|---|
| TOL | 50 | −7 | 12 | 19 |
| TMO | 24 | 40 | — | |
| PIN | 25 | 41 | 47 | |
| TOL | 85 | 25 | 41 | 47 |
| TMO | 21 | 35 | 38 | |
| PIN | 28 | 42 | 47 | |
| THF | 30 | 24 | 40 | 43 |
| MeTHF | 28 | 42 | 47 | |
| DMeTHF | 12 | 29 | 33 |
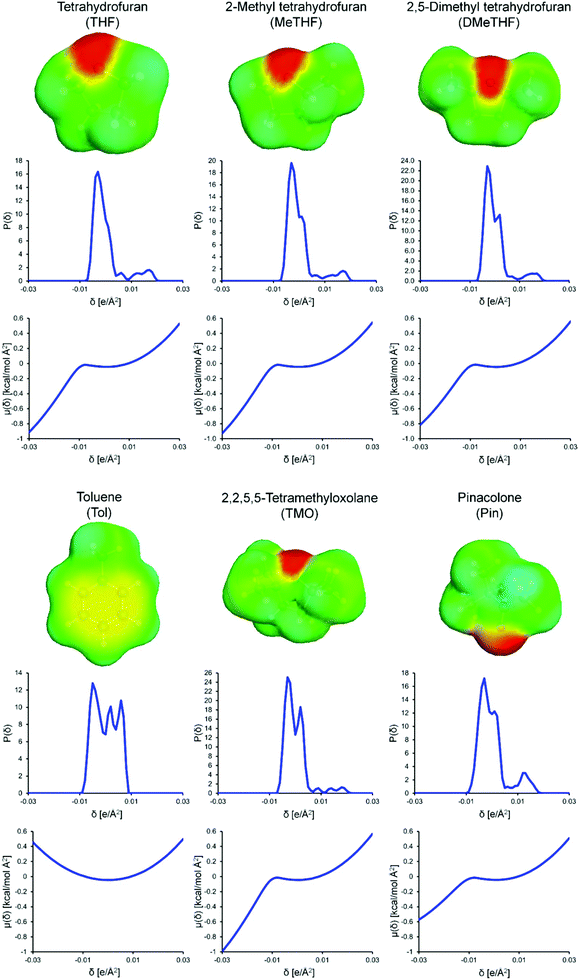
Computational investigations
Under otherwise identical conditions, the different monomer conversions and the resulting polymer molecular weights were found to depend on the type of solvent used. The solvent influences reaction rates, equilibria, solubility, and in this case, swelling of catalyst support (and hence the activity and stability of the catalyst). Computational tools were used to understand the role of the solvent, in particular, the COSMOtherm software package was used to calculate the σ-surfaces, σ-profiles and σ-potentials reported in Fig. 5. All theory related to COSMO-RS calculations and the COSMOtherm software can be found in previous literature.23–25 A free, web-based σ-profile database of 1432 compounds and a σ-profile database for predicting the solubility of solids in pure and mixed solvent mixtures are also available.26,27 In essence, COSMO-RS theory provides molecular surfaces representing the charge distribution in solvents (σ-surface). This can be converted to a histogram (σ-profile), and the interaction energy between a solvent and external charges (representing the surfaces of neighboring molecules, e.g. a solute) is the σ-potential.In this work, all the solvents are aprotic and therefore do not form specific interactions to stabilize negative charges. It can be observed that increasing the number of methyl substitutions on the THF motif reduces the relative proportion of electron donating surface area (Fig. 5). DMeTHF is less basic than the other ethers examined. Ketones have a more prominent oxygen atom compared to ethers in terms of the electronegative surface area of the molecule but are less basic; pinacolone follows this trend. Without heteroatoms, toluene has a hydrophobic profile. Aromatic solvents are polarizable whereas ethers and ketones have a permanent dipole.
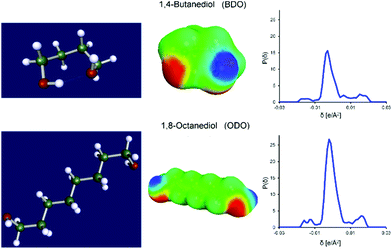
By modelling the diol reactants, 1,4-butanediol (Fig. 4, top) and 1,8-octanediol (Fig. 4, bottom), we observed that their lowest energy (and therefore most relevant) conformation are markedly different. 1,4-Butanediol preferentially forms an intramolecular hydrogen bond, while 1,8-octanediol does not. This suggests that in solution 1,8-octanediol is capable of two hydrogen bonds with either the solvent or potentially the ester reactant or another molecule of 1,8-octanediol. The intramolecular hydrogen bond of 1,4-butanediol reduces the dipole moment of the molecule, yet the reduction of electron density from the unbonded alcohol functionality increases its hydrogen bond donating ability towards a second, intermolecular hydrogen bond.
Hydrogen bonding and van der Waals force contributions to the chemical potential of species in solution can be separated to evaluate the specific interactions between solvent and diol and how this might influence the polymerization. COSMOtherm calculations at 30, 50, and 85 °C indicate 1,8-octanediol is more stable than 1,4-butanediol in all solvents, which may account for its reduced reactivity. At 50 °C, 25% of the energy of total solvent interaction between 1,4-butanediol (lowest energy conformation) and pinacolone is due to hydrogen bonding. This increases up to 35% with ether solvents. van der Waals forces are approximately equivalent for all solvents. Toluene is unable to engage in hydrogen bonding with 1,4-butanediol and so we would expect the intramolecular hydrogen bond of 1,4-butanediol to be preserved. In the oxygen-containing solvents it is possible to replace this interaction with an intermolecular hydrogen bond with the solvent. If this occurs prior to reaction, it may explain the difference in monomer conversion between toluene and the other solvents for the polymerization of dimethyl adipate at 50 °C (Fig. 1). At 85 °C, the entropic penalty of an intramolecular hydrogen bond creating a seven-membered ring structure may well negate the enthalpic benefit, restoring reactivity to that observed for TMO and pinacolone.
Materials and methods
Chemicals
Dimethyl adipate (DMA), 1,4-butanediol (BDO), toluene, and 1,8-octanediol (ODO) were purchased from Sigma Aldrich. Tetrahydrofuran (THF) 2-methyl tetrahydrofuran (2-MeTHF) and 2,5-dimethyltetrahydrofuran (DMeTHF) were purchased from TCI. Pinacolone (PIN) was purchased from Alfa Aesar. 2,5-Diethyl furandicarboxylate (DEF) was purchased from TCI. All other chemicals and solvents were purchased from Sigma-Aldrich and used as received if not otherwise specified. 2,2,5,5-Tetramethyloxolane (TMO) was synthesized and purified as previously reported.8Immobilized biocatalysts
Candida antarctica lipase B (CaLB) immobilized onto a macroporous acrylic resin also known as Novozym 435 (N435) was purchased from Sigma-Aldrich. Covalently immobilized CaLB (iCaLB, catalog code: IMMCALB-T2-150, resin IB-150A, activity: 2500 TBU g−1, 5000 PLU g−1) and cutinase from Aspergillus (aCuti, catalog code: IMML51-T1-350, lipase NZ 51032 (Cutinase1) adsorbed on acrylic beads, activity: 3000 PLU g−1) were purchased from Chiralvision (Leiden, The Netherlands). CaLB immo Plus (aCaLB, recombinant CaLB immobilized by adsorption on Purolite's highly hydrophobic carrier ECR1030M, activity: 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 PLU g−1) was a kind gift from c-LEcta (Leipzig, Germany). All preparations were vacuum dried for 48 h at 25 °C and stored in a desiccator prior to use.
300 PLU g−1) was a kind gift from c-LEcta (Leipzig, Germany). All preparations were vacuum dried for 48 h at 25 °C and stored in a desiccator prior to use.
Biocatalyzed polycondensation reactions in organic media
0.8 mmol of diester (A) and 0.8 mmol of diol (B) (diester![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diol ratio = 1.0
diol ratio = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0) and 4 mL of organic solvent were added to a 8 mL flat bottomed glass vial. The mixture was then stirred at 30, 50 or 85 °C until a homogeneous melt or dispersion of the compounds was obtained. Then, 20 mg of the immobilized enzyme preparation was added to the mixture and the reaction was magnetically stirred at 400 rpm for 6 h at ambient pressure. A Suba-Seal® vented with a syringe needle to avoid overpressure was used to seal the vial and avoid changes in the monomers concentration over time. After 6 h the biocatalyst was filtered off and the filtrate concentrated in vacuo. Reaction products were analyzed by 1H NMR spectroscopy and Gel Permeation Chromatography without any additional purification steps. The obtained polyesters were recovered in a range of forms going from viscous liquids to white solids depending on the used solvent, enzyme and operational conditions (see ESI, Fig. S14 and S15†).
1.0) and 4 mL of organic solvent were added to a 8 mL flat bottomed glass vial. The mixture was then stirred at 30, 50 or 85 °C until a homogeneous melt or dispersion of the compounds was obtained. Then, 20 mg of the immobilized enzyme preparation was added to the mixture and the reaction was magnetically stirred at 400 rpm for 6 h at ambient pressure. A Suba-Seal® vented with a syringe needle to avoid overpressure was used to seal the vial and avoid changes in the monomers concentration over time. After 6 h the biocatalyst was filtered off and the filtrate concentrated in vacuo. Reaction products were analyzed by 1H NMR spectroscopy and Gel Permeation Chromatography without any additional purification steps. The obtained polyesters were recovered in a range of forms going from viscous liquids to white solids depending on the used solvent, enzyme and operational conditions (see ESI, Fig. S14 and S15†).
1H nuclear magnetic resonance (1H NMR)
1H NMR spectroscopic analyses were performed on a JEOL JNM-ECS400A spectrometer at a frequency of 400 MHz. CDCl3 was used as the solvent. The reaction was monitored by collecting the 1H-NMR spectra of the crude reaction product (see ESI, Fig. 13† for a fully assigned spectra) The monomer conversion was calculated using the ratio between the 1H signals at δ 3.68 (t, 2H, –CH2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) and at δ 4.10 (t, 2H, –C
2–OH) and at δ 4.10 (t, 2H, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2OC(O)).
2OC(O)).
Gel permeation chromatography (GPC)
Polymeric samples were diluted in THF at a concentration of ∼2 mg mL−1. GPC runs were carried out using a PSS SDV high set composed of 3 analytical columns (300 × 8 mm, particle diameter 5 μm) of 1000, 105 and 106 Å pore sizes with a PSS guard column (Polymer Standards Service GmbH) installed in a PSS SECcurity SEC system. Elution was carried out using BHT-inhibited THF as the mobile phase (1 mL min−1). Column temperature was maintained at 23 °C and detection was performed by refractive index detector. The elution time was 50 min. Calibration was carried out in the molecular weight range 370–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520
520![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da using the ReadyCal polystyrene standards supplied by Sigma Aldrich. Toluene was added to each sample as internal standard.
000 Da using the ReadyCal polystyrene standards supplied by Sigma Aldrich. Toluene was added to each sample as internal standard.
Differential scanning calorimetry (DSC)
Modulated DSC analysis were performed on a TA Instruments Q2000 under nitrogen atmosphere. The used heating rate was of 5 °C min−1 over a T range of −60–200 °C. Cooling rate was set at 5 °C min−1 over the same T range. Sample mass was of 5–10 mg for all measured samples. The melting points of the polymers were calculated from the second heating scan.Thermogravimetric analysis (TGA)
TGA was performed on a PL Thermal Sciences STA 625 thermal analyzer. ∼10 mg of sample was weighted in an aluminium pan. The sample was then placed into the furnace with a N2 flow of 100 mL min−1 and heated from 25 to 625 °C at a heating rate of 10 °C min−1. From the TGA profiles the temperatures at 5, 10 and 50% mass loss (Td 5, 10 and 50) were obtained.Computational studies
ArgusLab (version 4.0.1, Mark Thompson and Planaria Software LLC, 2004) was used to draw 3D models of the compounds. Conformations of the molecules were then calculated with COSMOconfX (version 4.0, COSMOlogic GmbH & Co. KG, 2015). Chemical potentials were generated with COSMOthermX (version C30_1705, COSMOlogic GmbH & Co. KG, 2017). The charge density found on the surface of each solvent is calculated by COSMOthermX to produce a 3D representation, which then determines the corresponding sigma profile and chemical potential plot.Conclusions
In the present work we present the improved enzyme-catalysed synthesis of adipate and furandicarboxylate polymers using four green solvents compared to the traditional solventless bulk process. The green solvents tested were 2-methyltetrahydrofuran, 2,5-dimentyltetrahydrofuran, 2,2,5,5-tetramethyloxolane and pinacolone. The traditional solvents, toluene and THF, were also used for comparison.Pinacolone was demonstrated to be the best solvent for this process, producing superior polymers with higher molecular masses (Mn >2000 Da and conversions of over 80%) at significantly lower temperatures than the traditional solventless process (30 or 50 °C compared to 85 °C).
Author contributions
A. P., M. V. and F. P. B. performed the enzymatic polymer synthesis and the materials characterization. A. P. and J. S. performed the computational analysis. A. P. and J. W. C. performed the thermal analysis. A. P. and F. P. B. planned the experiments. A. P., J. S., J. W. C. and F. P. B. wrote the manuscript. A. P., F. P. B. and T. J. F. supervised the work. All authors corrected the manuscript and discussed the data prior to submission.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
A. P. thanks the FWF Erwin Schrödinger fellowship (Grant agreement J4014-N34) for financial support. The authors thank Biome bioplastics for financial support. COSMO-RS calculations are courtesy of, and authorised for the purposes of, Circa Group Pty Ltd. This publication also includes results from the ReSolve project. This project has received funding from the Bio-Based Industries Joint Undertaking under the European Union's Horizon2020 research and innovation programme under agreement No 745450. The publication reflects only the authors’ view and the JU is not responsible for any use that may be made of the information it contains.Notes and references
- US EPA – Initial List of Hazardous Air Pollutants with Modifications, https://www.epa.gov/haps/initial-list-hazardous-air-pollutants-modifications (accessed October 2018).
- n-Hexane – Substance Information – ECHA, https://echa.europa.eu/substance-information (accessed October 2018).
- Toluene – Substance Information – ECHA, https://echa.europa.eu/substance-information/-/substanceinfo/100.003.297 (accessed October 2018).
- n-Hexane – Substance evaluation – CoRAP – ECHA, https://echa.europa.eu/information-on-chemicals/evaluation/community-rolling-action-plan/corap-table/-/dislist/details/0b0236e1807e4bd4 (accessed October 2018).
- A. R. Alcantara and P. D. de Maria, Curr. Green Chem., 2018, 5, 86–103 CrossRef CAS.
- V. Antonucci, J. Coleman, J. B. Ferry, N. Johnson, M. Mathe, J. P. Scott and J. Xu, Org. Process Res. Dev., 2011, 15, 939–941 CrossRef CAS.
- V. Pace, P. Hoyos, L. Castoldi, P. D. De María and A. R. Alcántara, ChemSusChem, 2012, 5, 1369–1379 CrossRef CAS PubMed.
- F. Byrne, B. Forier, G. Bossaert, C. Hoebers, T. J. Farmer, J. H. Clark and A. J. Hunt, Green Chem., 2017, 19, 3671–3678 RSC.
- F. Byrne, B. Forier, G. Bossaert, C. Hoebers, T. J. Farmer and A. J. Hunt, Green Chem., 2018, 20, 4003–4011 RSC.
- A. Pellis, S. Cantone, C. Ebert and L. Gardossi, New Biotechnol., 2018, 40(Part A), 154–169 CrossRef CAS PubMed.
- Y. Jiang, D. Maniar, A. J. J. Woortman and K. Loos, RSC Adv., 2016, 6, 67941–67953 RSC.
- L. Corici, V. Ferrario, A. Pellis, C. Ebert, S. Lotteria, S. Cantone, D. Voinovich and L. Gardossi, RSC Adv., 2016, 6, 63256–63270 RSC.
- P. D. De María, J. V. Sinisterra, J. M. Sánchez-Montero, M. Lotti, F. Valero and A. R. Alcántara, Enzyme Microb. Technol., 2006, 38, 199–208 CrossRef.
- A. Pellis, A. Guarneri, M. Brandauer, E. Herrero Acero, H. Peerlings, L. Gardossi and G. M. Guebitz, Biotechnol. J., 2016, 11, 642–647 CrossRef CAS PubMed.
- C. Fodor, M. Golkaram, A. J. J. Woortman, J. van Dijken and K. Loos, Polym. Chem., 2017, 8, 6795–6805 RSC.
- G. Paggiola, A. J. Hunt, C. R. McElroy, J. Sherwood and J. H. Clark, Green Chem., 2014, 16, 2107–2110 RSC.
- A. Iemhoff, J. Sherwood, C. R. McElroy and A. J. Hunt, Green Chem., 2018, 20, 136–140 RSC.
- A. Pellis, E. Herrero Acero, L. Gardossi, V. Ferrario and G. M. Guebitz, Polym. Int., 2016, 65, 861–871 CrossRef CAS.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehad and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- A. Pellis, J. W. Comerford, A. J. Maneffa, M. H. Sipponen, J. H. Clark and T. J. Farmer, Eur. Polym. J., 2018, 106, 79–84 CrossRef CAS.
- S. Weinberger, A. Pellis, J. W. Comerford, T. J. Farmer and G. M. Guebitz, Catalysts, 2018, 8, 369 CrossRef.
- A. Pellis, L. Corici, L. Sinigoi, N. D'Amelio, D. Fattor, V. Ferrario, C. Ebert and L. Gardossi, Green Chem., 2015, 17, 1756–1766 RSC.
- A. Klamt, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2018, 8, e1338 Search PubMed.
- COSMOtherm, Version C3.0, Release 17.01, COSMOlogic GmbH & Co. KG, http://www.cosmologic.de Search PubMed.
- F. Eckert and A. Klamt, AIChE J., 2002, 48, 369 CrossRef CAS.
- E. Mullins, R. Oldland, Y. A. Liu, S. Wang, S. I. Sandler, C.-C. Chen, M. Zwolak and K. C. Seavey, Ind. Eng. Chem. Res., 2006, 45, 4389–4415 CrossRef CAS.
- E. Mullins, Y. A. Liu, A. Ghaderi and S. D. Fast, Ind. Eng. Chem. Res., 2008, 47, 1707–1725 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H-NMR spectra and conversions, GPC-calculated number average molecular weights, weight average molecular weights, dispersity data, products photographs and molecular modelling data. See DOI: 10.1039/c8gc03567a |
| This journal is © The Royal Society of Chemistry 2019 |