Ultrasound assisted ZnO coating in a microflow based photoreactor for selective oxidation of benzyl alcohol to benzaldehyde†
Abstract
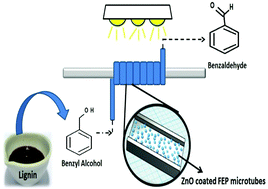
Heterogeneous photocatalysis in a microflow system for generation of value added chemicals is a novel green chemistry approach requiring the understanding of photocatalysis, microfluidics and reactor design. Research on the development of a low energy and environmentally friendly based photo-microreactor system for photocatalysis is yet to be explored. Commercial ZnO nanoparticles were deposited in the inner wall of a fluoropolymer using a low energy ultrasound bath under mild conditions and later used for selective conversion of aromatic alcohols to aldehydes. The deposition of the nanoparticles on the fluoropolymer is mainly attributed to the physical changes taking place inside the microtubes under the effect of ultrasound.
- This article is part of the themed collection: Green Biorefinery Technologies based on Waste Biomass


 Please wait while we load your content...
Please wait while we load your content...