Open Access Article
Open Access ArticleIntestinal anti-inflammatory effects of artichoke pectin and modified pectin fractions in the dextran sulfate sodium model of mice colitis. Artificial neural network modelling of inflammatory markers†
Carlos
Sabater
a,
Jose Alberto
Molina-Tijeras
b,
Teresa
Vezza
b,
Nieves
Corzo
a,
Antonia
Montilla
 *a and
Pilar
Utrilla
b
*a and
Pilar
Utrilla
b
aInstituto de Investigación en Ciencias de la Alimentación CIAL, (CSIC-UAM) CEI (UAM+CSIC), C/Nicolás Cabrera, 9, E-28049 Madrid, Spain. E-mail: a.montilla@csic.es; Tel: +34 910017952
bDepartamento de Farmacología, Centro de Investigaciones Biomédicas en Red – Enfermedades Hepáticas y Digestivas (CIBER-EHD), Centro de Investigación Biomédica, Universidad de Granada, Granada, Spain
First published on 31st October 2019
Abstract
Anti-inflammatory properties of artichoke pectin and modified fractions (arabinose- and galactose-free) used at two doses (40 and 80 mg kg−1) in mice with colitis induced by dextran sulfate sodium have been investigated. Expression of pro-inflammatory markers TNF-α and ICAM-I decreased in groups of mice treated with original and arabinose-free artichoke pectin while IL-1β and IL-6 liberation was reduced only in mice groups treated with original artichoke pectin. A decrease in iNOS and TLR-4 expression was observed for most treatments. Intestinal barrier gene expression was also determined. MUC-1 and Occludin increased in groups treated with original artichoke pectin while MUC-3 expression also increased in arabinose-free pectin treatment. Galactose elimination led to a loss of pectin bioactivity. Characteristic expression profiles were established for each treatment through artificial neural networks showing high accuracy rates (≥90%). These results highlight the potential amelioration of inflammatory bowel disease on mice model colitis through artichoke pectin administration.
1. Introduction
Pectin is widely used as a functional ingredient in the industry due to its technological properties. However, in recent years, its biological properties have gained attention.1 This complex polysaccharide is mainly composed of a linear chain of galacturonic acid (GalA) units bonded by α(1,4) linkages called homogalacturonan (HG), which comprises approximately 65% of pectin and may be partially methyl-esterified at the O-6 carboxyl and may be O-acetylated at O-2 or O-3. Other major domains of pectin are rhamnogalacturonan-I (RG-I) and RG-II. RG-I comprises 20–35% of the pectin structure and is made of sequences of disaccharide [-4)-α-D-GalpA-(1,2)-α-L-Rhap-(1-]n. Rhamnose (Rha) may be substituted at O-4 with linear or branched oligosaccharides of arabinose (arabinans), galactose (galactans) or both (arabinogalactans) showing degrees of polymerisation (DP) up to 47 units depending on the pectin source.2 RG-II constitutes a minor and more complex domain which comprises 10% of pectin. This domain consists of 12 different types of sugars in over 20 different linkages and is largely conserved across plant species.3 Pectin technological and biological properties are determined by its structural characteristics such as the monomeric composition, molecular weight (Mw), presence and distribution of side chains, degree of methyl-esterification (DM, % methyl lesterification GalA) and acetylation, and charge distribution along their backbone.4Several studies evaluated the in vivo bioactivity of pectin including its non-pharmacological administration to ameliorate the symptoms of inflammatory bowel disease (IBD).5,6 One of the most studied models for investigating the pathogenesis of IBD is the dextran sulfate sodium (DSS)-induced colitis mice model characterized by bloody faeces, diarrhoea, weight loss and tissue inflammation. Expression of pro-inflammatory cytokines (IL-1β, TNF-α and IL-6) that may impair intestine permeability and mucosal barrier function is also increased, leading to intestine inflammation.7 It has been reported that diets containing citrus pectin and probiotics (Bifidobacterium longum) ameliorated the symptoms of acute and chronic DSS-induced colitis in mice.8 Likewise, diets containing dietary pectin and inulin reduced intestinal inflammation and cancer incidence in mice.9
Efforts have been made to correlate pectin structural characteristics with its anti-inflammatory effect. Polysaccharides from ginseng with high GalA, galactose and arabinose contents suppressed pro-inflammatory cytokine expression in mice,10 and soluble dietary fiber containing pectic substances, with high GalA contents and DM, reduced gastric ulcer lesions in rats by 87%.11 Bioactivity of specific pectin domains or modified pectin fractions has also been studied. For example, galacturonan from starfruits showing high contents of arabinans and arabinogalactans exerted antinociceptive and anti-inflammatory properties in mice,12 and enzymatically modified apple polysaccharides, using pectinases, may protect against colitis associated colorectal cancer in mice.13 Moreover, it has been demonstrated that arabinose and galactose contents may determine the biological activity of pectin.14,15 In this sense, pectin from artichoke by-products, because of its high content of arabinose (127 mg g−1 of pectin) and galactose (24 mg g−1 of pectin),16 can be an interesting natural product to analyse its anti-inflammatory properties.
On the other hand, data modelling allows discovering valuable information on complex chemical and biological events. With this aim, machine learning algorithms like artificial neural networks (ANN) have been recently applied to characterize artichoke pectin chains extracted by different methods,17 and to determine complex structural patterns of pectic oligosaccharides obtained from different pectin sources.18,19 In addition, these advanced data analysis tools might be employed to develop predictive models of disease course and response to therapy, as well as for characterization of disease heterogeneity and drug development for IBD.20
Therefore, the aim of this study was to investigate the anti-inflammatory effect of artichoke pectin (AP) and modified artichoke pectin fractions (without arabinose, APwA; or galactose, APwG) in a experimental DSS model of colitis in mice. Then, specific patterns in cytokine and intestinal protein expression were established for each type of sample through ANN modelling.
2. Materials and methods
2.1. Analytical standards and reagents
Analytical reference standards such as D-xylose, L-arabinose, L-rhamnose, D-galactose, galacturonic acid (GalA) and phenyl-β-glucoside were purchased from Sigma Aldrich (Steinheim, Germany). Viscozyme®L (endo-1,3(4)-β-glucanase from Aspergillus aculeatus) was a generous gift from Novozymes (Bagsvaerd, Denmark). β-Galactosidase from Bacillus circulans and endo-1,5-α-arabinanase from Aspergillus niger were acquired from Biocon (Barcelona, Spain) and Megazyme (Bray, Ireland), respectively. Citrus pectin (CP) was acquired from CEAMSA (O'Porriño, Spain). DSS (36–50 kDa) was purchased from MP Biomedicals (Santa Ana, CA, USA). RNAlater® was obtained from Sigma Aldrich (Steinheim, Germany), and Tri-Reagent® was acquired from Thermo Fisher Scientific (Invitrogen, USA). The oligo (dT) primers (Promega, Southampton, UK) and KAPA SYBRsFAST qPCR Master Mix (Kapa Biosystems, Inc., Wilmington, MA, USA) were used to perform the qPCR analyses. Ultrapure water (18.2 MΩ cm, with levels of 1–5 ng mL−1 total organic carbon and <0.001 EU mL−1 pyrogen) produced in-house with a laboratory water purification system (Milli-Q Synthesis A10, Millipore, Billerica, MA, USA) was used throughout.2.2. Obtainment and analysis of modified artichoke pectin
Artichoke pectin (AP) was previously extracted in our laboratory using enzymatic preparation Celluclast®1.5L.16 Galactose-free pectin (APwG) was obtained by enzymatic hydrolysis of 2% (w/v) AP solutions dissolved in 0.05 M acetate buffer (pH 5.0) with 0.7 U mL−1 of β-galactosidase from B. circulans at 50 °C for 5 h. Similarly, arabinose-free pectin (APwA) was obtained by enzymatic hydrolysis of 2% (w/v) AP solutions dissolved in 0.2 M acetate buffer (pH 4.0) with 0.4 U mL−1 of endo-1,5-α-arabinanase from A. niger at 50 °C for 24 h. Samples were immediately immersed in boiling water to inactivate the enzyme. Then, modified pectins were purified using an Ultracel® ultrafiltration (UF) membrane (Mw cut-off 1 kDa; Millipore, Billerica, MA, USA). Retentates were freeze-dried for subsequent analysis.2.3. Analytical determinations
To determine the monomeric composition of purified APwG and APwA, samples (2% w/v in 0.05 M sodium acetate buffer; pH 5.0) were hydrolysed with 90 U mL−1 of Viscozyme®L preparation and then sugars released were analysed as TMSO by GC-FID, following the method previously described by Sabater et al.16 On the other hand, complete Mw distribution profiles of artichoke modified pectins were determined by HPSEC-ELSD.16,18 The DM was determined by FT-IR.16,17 All analyses were carried out in duplicate.2.4. Experimental design
Potential anti-inflammatory properties in experimental colitis mice of original AP, APwG and APwA were compared to those of CP, which presents recognized anti-inflammatory activity. This study was carried out in accordance with the Guide for the Care and Use of Laboratory Animals as promulgated by the National Institute of Health and the protocols were approved by the Ethic Committee of Animal Experimentation of the University of Granada (Spain) (Ref. No. CEEA 2010-286). Male C57BL/6 mice (7–9 weeks old; 23 ± 2 g) were purchased from Janvier (St Berthevin Cedex, France). Mice were housed in Makrolon cages, maintained under an air-conditioned atmosphere with a 12 h light–dark cycle, and provided free access to tap water and a standard rodent diet (Panlab A04 diet, Panlab S.A., Barcelona, Spain). Mice were maintained under specific pathogen-free conditions in the facilities of the University of Granada and were randomly assigned to ten groups of six animals each (Table 1): (1) healthy, (2) DSS treated, (3) DSS treated with: CP 40 mg kg−1, (4) CP 80 mg kg−1, (5) AP 40 mg kg−1, (6) AP 80 mg kg−1, (7) APwA 40 mg kg−1, (8) APwA 80 mg kg−1, (9) APwG 40 mg kg−1, and (10) APwG 80 mg kg−1. Induction of colitis was performed 15 days after the start of the experiment by adding 3.0% (w/v) DSS to the drinking water for five days (Table 1). Pectin fractions were diluted with water and administered by oral gavage (100 μL per day) corresponding to doses of 40 and 80 mg kg−1. Animal body weight, the presence of gross blood in the faeces and the stool consistency were registered to calculate the Disease Activity Index (DAI) (ESI Table S1†). Once the animals were sacrificed, the colon was aseptically removed and washed and then weighed. The ratio between the colon weight and length was also considered as a macroscopic indicator. Then, the expressions of pro-inflammatory cytokines and barrier intestinal proteins were evaluated. Total RNA from colonic samples was isolated, reverse transcribed and amplified by qPCR using specific primers (Table 2). mRNA expression was normalized using the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal control. The mRNA relative was calculated using the ΔΔCt method.| Mice groups (n = 6) | Animal weight (kg) | Daily ingredient dose of treatment (mg per kg of body weight) | DSS supply (days) | Treatment supply (days) |
|---|---|---|---|---|
| Healthy | 0.023 ± 0.002 | 0 | — | — |
| DSS treated | 0.022 ± 0.002 | 0 | 5 | — |
| DSS treated + CP 40 | 0.023 ± 0.002 | 40 | 5 | 15 |
| DSS treated + CP 80 | 0.023 ± 0.001 | 80 | 5 | 15 |
| DSS treated + AP 40 | 0.023 ± 0.001 | 40 | 5 | 15 |
| DSS treated + AP 80 | 0.023 ± 0.002 | 80 | 5 | 15 |
| DSS treated + APwA 40 | 0.023 ± 0.002 | 40 | 5 | 15 |
| DSS treated + APwA 80 | 0.023 ± 0.001 | 80 | 5 | 15 |
| DSS treated + APwG 40 | 0.022 ± 0.002 | 40 | 5 | 15 |
| DSS treated + APwG 80 | 0.023 ± 0.001 | 80 | 5 | 15 |
| Gene | Sequence 5′-3′ | Annealing T (°C) |
|---|---|---|
| GAPDH | FW 5′-CCATCACCATCTTCCAGGAG-3′ | 60 |
| RV 5′-CCTGCTTCACCACCTTCTTG-3′ | ||
| TNF-α | FW 5′-AACTAGTGGTGCCAGCCGAT-3′ | 60 |
| RV 5′-CTTCACAGAGCAATGACTCC-3′ | ||
| IL-1β | FW 5′-TGATGAGAATGACCTGTTCT-3′ | 60 |
| RV 5′-CTTCTTCAAAGATGAAGGAAA-3′ | ||
| IL-6 | FW 5′-TAGTCCTTCCTACCCCAATTTCC-3′ | 60 |
| RV 5′-TTGGTCCTTAGCCACTCCTTCC-3′ | ||
| iNOS | FW 5′-GTTGAAGACTGAGACTCTGG-3′ | 67 |
| RV 5′-ACTAGGCTACTCCGTGGA-3′ | ||
| ICAM-1 | FW 5′-CAGTCCGCTGTGCTTTGAGA-3′ | 60 |
| RV 5′-CGGAAACGAATACACGGTGAT-3′ | ||
| TLR-4 | FW 5′-GCCTTTCAGGGAATTAAGCTCC-3′ | 60 |
| RV 5′-AGATCAACCGATGGACGTGTAA-3′ | ||
| MUC-1 | FW 5′-GCAGTCCTCAGTGGCACCTC-3′ | 60 |
| RV 5′-CACCGTGGGCTACTGGAGAG-3′ | ||
| MUC-2 | FW 5′-GCTGACGAGTGGTTGGTGAAT-3′ | 60 |
| RV 5′-GATGAGGTGGCAGACAGGAGA-3′ | ||
| MUC-3 | FW 5′-CGTGGTCAACTGCGAGAATGG-3′ | 60 |
| RV 5′-CGGCTCTATCTCTACGCTCTCC-3′ | ||
| Occludin | FW 5′-ACGGACCCTGACCACTATGA-3′ | 60 |
| RV 5′-TCAGCAGCAGCCATGTACTC-3′ | ||
| ZO-1 | FW 5′-GGGGCCTACACTGATCAAGA-3′ | 56 |
| RV 5′-TGGAGATGAGGCTTCTGCTT-3′ | ||
| TFF-3 | FW 5′-CCTGGTTGCTGGGTCCTCTG-3′ | 60 |
| RV 5′-GCCACGGTTGTTACACTGCTC-3′ | ||
| Villin | FW 5′-CTCCGAGCAGATTGAGAAGG-3′ | 60 |
| RV 5′-GGTGCTGCCACTCTTCTACC-3′ |
2.5. Data analysis
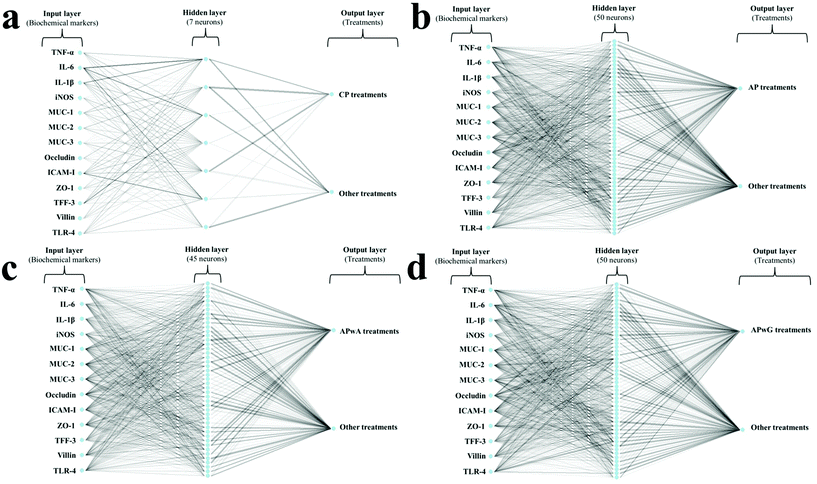
ANOVA tests and Tukey's test for p < 0.05 were applied to all data generated. In order to establish reproducible patterns in the expression of cytokines and intestinal proteins for each group of mice treated with pectins, artificial neural network (ANN) models were developed. ANNs are powerful pattern-recognition algorithms and the most common type of ANN is the multilayer perceptron. This model is formed by an input layer (i.e. biochemical markers), an output layer (i.e. treatment of mice groups) and several neurons or nodes organised in hidden layers, where each neuron in a layer is connected with each neuron in the next layer through a weighted connection. In addition, a mathematical transformation (activation function) is applied to the input layer to determine whether the information that the neuron is receiving is relevant or not. Six ANNs were computed to determine specific patterns for all studied samples (both doses were included in the same sample type group): (1) healthy control (ANN-1); (2) DSS control (ANN-2); (3) CP (ANN-3); (4) AP (ANN-4); (5) APwA (ANN-5); (6) APwG (ANN-6). Fig. 1 shows the architecture of ANN used to determine patterns in pectin and modified pectin treatments. The numbers of neurons in the hidden layer were 1, 5, 7, 50, 45 and 50 for ANN-1, -2, -3, -4, -5 and -6, respectively. The number of neurons in the hidden layer depends on the complexity of the input data (i.e. expression levels of biochemical parameters). The number of neurons is one of the most important parameters that need to be adjusted in an ANN model to ensure its predictive power. This process is called “parameter tuning”. One of the most common methods to find the optimal number of neurons for each model is called “grid search”. In this method, a range of values for each parameter that needs to be tuned is set (i.e. number of neurons 1–100) and then, a model for every combination of the parameter values is built. Once the performance of each combination is evaluated, the optimal model is selected. The activation function for ANN-1, -2 and -3 was logistic while rectifier, tanh and maxout functions were used for ANN-4, -5 and -6, respectively. Variables were scaled and centered before computing the analyses. All the models were trained with 70% of the data, 10-fold cross-validated and then tested with 30% of data from each class (corresponding to new samples).A variable importance analysis was carried out. The coefficients for each parameter (i.e. biochemical marker), were calculated as the sum of the products of raw input-hidden and hidden-output connection weights. These connection weights are represented in Fig. 1 as lines connecting the input layer (i.e. biochemical markers), the hidden layer (neurons) and the output layer (i.e. type of treatment). Each biochemical marker was expressed as the relative values, corresponding to variable importance coefficients divided by the highest variable importance value and multiplied by 100, so that values are bounded between 0 and 100.
All statistical analyses were computed on R v3.5.0. ANN models were built using RSNNS and H2O packages.21,22
3. Results
3.1. Characterization of artichoke pectin and modified pectin fractions
Characterization of CP and AP as well as modified artichoke pectin fractions (APwG and APwA) purified by UF has been performed in order to know structural differences and thus to assess their influence on each group of mice studied. Enzymatic treatment of AP with β-galactosidase from B. circulans and endo-1,5-α-arabinanase from A. niger produced galactose-free (APwG) and arabinose-free (APwA) modified artichoke pectin, respectively. Galactose release during enzymatic hydrolysis was calculated considering the galactose contents of initial AP (2.4 ± 0.0 mg 100 mg−1 dry matter of pectin) and final of APwG (0.3 ± 0.0 mg 100 mg−1 dry matter of pectin) leading to a loss of 87.5 ± 1.1% of total galactose present in AP. Similarly, arabinose release during enzymatic hydrolysis was calculated considering the arabinose contents of initial AP (12.7 ± 0.6 mg 100 mg−1 dry matter of pectin) and final of APwA (1.5 ± 0.2 mg 100 mg−1 dry matter of pectin) leading to a loss of 88.2 ± 1.4% of total arabinose present in AP. The monosaccharide composition of AP, CP and modified pectins was determined and expressed as percentages of total carbohydrates (ESI Table S2†). Galactose content in APwG comprised 0.6% of total carbohydrates while arabinose content in APwA comprised 1.4% of total carbohydrates. As expected, modified artichoke pectins had a slightly higher GalA percentage (76–77%) than the original pectin chain and APwA showed significantly higher proportions of rhamnose (6.3%) than AP (ESI Table S2†), highlighting that the linear backbone of HG and RG-I was preserved. Xylose content was significantly higher in APwG (1.5%). In contrast, commercial CP used as the control showed significantly higher xylose and galactose contents (2.5 and 11.1%) and lower arabinose contents than AP. In addition, different ratios (degree of branching (Rha/GalA), linearity pectin backbone [GalA/(Rha + Ara + Gal)] and extent of branching of RG-I [(Ara + Gal)/Rha]) were calculated,16,17 showing primary structural properties of pectin molecules, based on pectin monosaccharide composition data (ESI Table S2†). Modified AP fractions showed a higher linearity pectin backbone than original AP, although these differences were not statistically significant. In contrast, APwA showed a significantly lower extent of branching of RG-I than APwG, indicating the removal of side chains during enzymatic modification. These coefficients between contents of GalA and neutral pectic sugars indicate possible branching along the pectin molecules.Another important structural characteristic is DM, so CP shows higher DM (71%) than AP (19.5%) and modified pectins. On the other hand, APwG showed a multimodal Mw distribution similar to original AP (ESI Table S3†) with three main fragments of 605, 74 and 5.1 kDa. In contrast, APwA had a bimodal distribution showing two main fragments of 542 and 63 kDa. It should be noted that CP used as the control showed a monomodal distribution with one main fragment of 547 kDa.
3.2. Influence of pectin treatment on macroscopic indicators of inflammatory bowel disease in DSS -treated mice
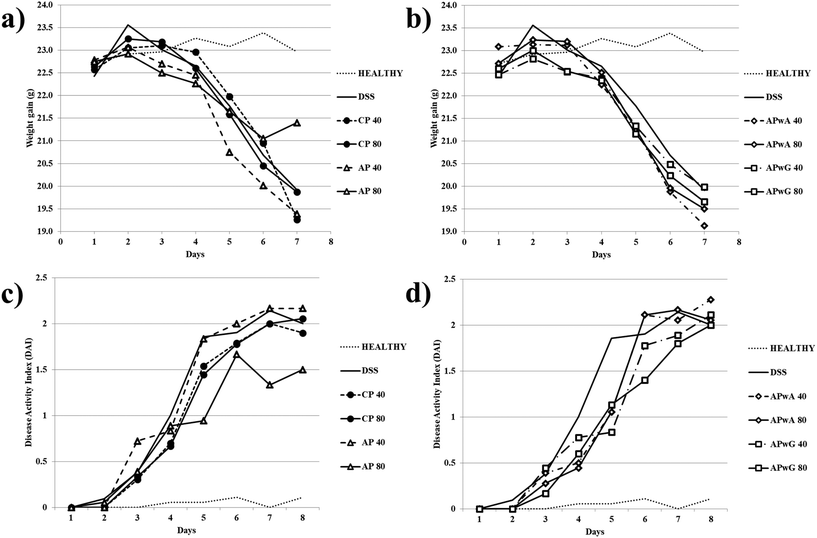
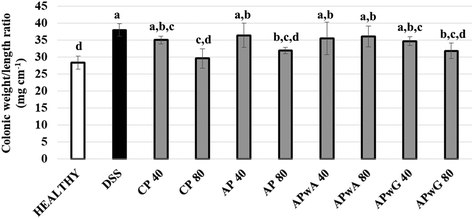
First, different macroscopic indicators and symptoms of colitis induced in mice by the oral administration of DSS were studied. A significant weight loss was observed in all experimental groups except for the healthy control group (Fig. 2a and b). As expected, DSS-induced colitis produced a weight loss in mice due to a systemic status of illness occasioned by DSS as a general toxic. In general, weight losses in mice treated with modified AP were similar to those of the DSS control group. The only positive effect was observed after the administration of original AP at doses of 80 mg kg−1, resulting in a lower weight reduction than CP with recognized anti-inflammatory activity used as the control. In the AP 80 group, no weight losses were observed at the seventh day of treatment. Disease progression was monitored by calculating the DAI for each group (Fig. 2c and d). AP at doses of 80 mg kg−1 resulted in a significant decrease of the DAI value on the seventh and eighth days compared to the sixth; these values were lower than the one of CP at the same doses in agreement with high weight loss values. No significant differences in the DAI compared to the DSS control were observed for the rest of the treatments.On the other hand, the weight/length ratio of the colon was determined (Fig. 3). This ratio reached its highest and lowest values in DSS and healthy control groups, respectively. A statistically significant decrease in this ratio (p < 0.05) with respect to the DSS control group was only obtained for CP, AP and APwG at doses of 80 mg kg−1. These results may highlight a lower severity of inflammation and minor colonic cell infiltration in these groups (CP 80, AP 80 and APwG 80). These results were in agreement with those of Pacheco et al.6 who observed a significant reduction of IBD symptoms and improvement of the animals’ general status in mice treated with this CP. However, differences between the same product administered at different doses (40 and 80 mg kg−1) were not statistically significant. In contrast, no significant differences were found between the DSS group, the groups intake 40 mg kg−1 dose of all ingredients and also APwA at 80 mg kg−1.
Considering this set of macroscopic indicators, an anti-inflammatory effect for original AP is shown leading to a significant reduction of symptoms. However, enzymatic modification of AP did not enhance its bioactivity.
3.3. Influence of pectin treatment on inflammatory biochemical markers in DSS-treated mice corroborated by ANN modelling
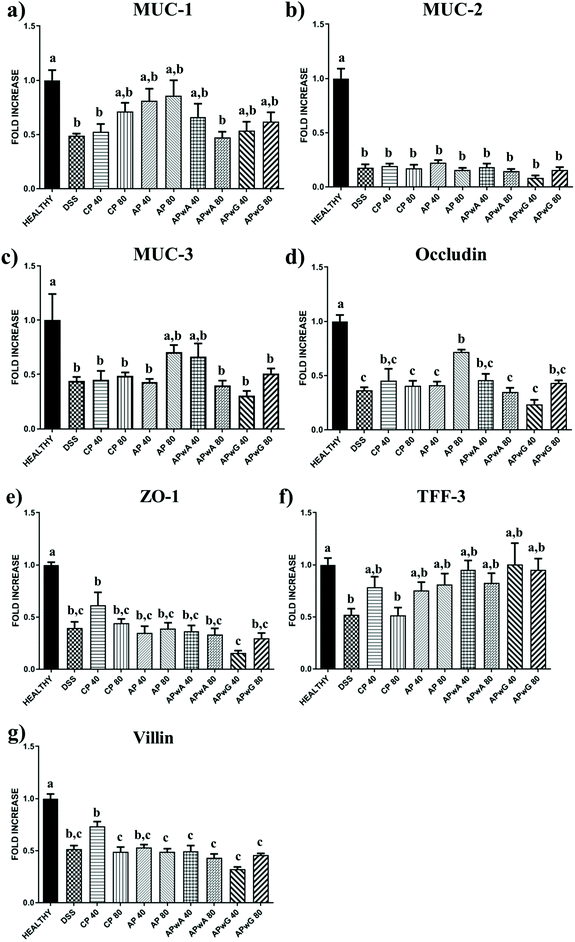
To gain deeper knowledge of the disease progression in mice, several biochemical markers of IBD were assessed (Fig. 4 and 5). This set of markers consisted of several pro-inflammatory parameters such as cytokines (TNF-α, IL-1β, IL-6), (Fig. 4) enzymes (iNOS) and transmembrane receptors (ICAM-I, TLR-4; Fig. 4); as well as different epithelial integrity markers such as barrier intestinal proteins (MUC-1, MUC-2, MUC-3, Occludin, ZO-1, TFF-3, Villin) (Fig. 5). Given the great variability observed between individuals, ANN models were trained on biochemical parameters to obtain more robust and confident expression patterns for each group of mice studied (healthy, DSS treated, and DSS treated and supplemented with CP, AP, APwA and APwG) which may help to interpret these results. These models had different architectures as it can be seen in Fig. 1. The optimal number of neurons increases with input data complexity. The ANN-1 model for the healthy control had only 1 hidden neuron because this group is the easiest to discriminate from the rest of the groups based on biochemical markers, which were not altered. Similarly, the ANN-2 model for DSS control is built with a small number of neurons because this group shows the highest pro-inflammatory cytokine levels and the lowest barrier protein expression values. The ANN-3 model for CP control also had a small number of neurons probably due to the accentuated effect of CP on some parameters like iNOS or Villin, so this type of pectin is easily differentiated from the rest of the treatments. In contrast, AP and modified AP fractions administered at both doses exerted similar effects on some of the biochemical parameters and it was more difficult to discriminate these treatments. The expression profiles for these samples are more complex than the ones observed for the controls and therefore, ANN models with a larger number of neurons are needed.These algorithms found a reproducible classification pattern for each kind of substrate (ESI Table S4†), showing high prediction rates on the independent test set, above 90% in all cases. Kappa, a more robust accuracy metric that considers the possibility of a correct classification by chance, showed the lowest values for APwA and original AP probably due to the highest differences observed between the effects of these samples at the two studied doses (Fig. 4 and 5). However, kappa values were above 0.80, indicating a high reproducibility. Therefore, the ANN was able to find general patterns that allow discriminating the biochemical profile obtained for each group of mice studied considering these high prediction rates, sensitivity, specificity and balanced accuracy of these models (above 90%) (ESI Table S4†). To interpret ANN results, the importance of each biochemical parameter in these models was calculated (Table 3). Differences observed in cytokine expression profiles, based on this analysis, are discussed below.
| Parameter | ANN-1 (healthy) | DSS treatment | ||||
|---|---|---|---|---|---|---|
| ANN-2 (control) | ANN-3 (+CP) | ANN-4 (+AP) | ANN-5 (+APwA) | ANN-6 (+APwG) | ||
| TNF-α | 3.8 | 34.0 | 46.3 | 69.3 | 96.1 | 81.8 |
| IL-6 | 12.5 | 15.7 | 100 | 100 | 92.4 | 100 |
| IL-1β | 13.6 | 15.0 | 32.3 | 63.8 | 59.4 | 61.4 |
| iNOS | 5.4 | 3.6 | 80.8 | 63.1 | 82.9 | 77.4 |
| ICAM-I | 8.2 | 100 | 9.6 | 71.5 | 100 | 92.1 |
| TLR-4 | 23.9 | 53.7 | 24.7 | 75.9 | 87.3 | 86.4 |
| MUC-1 | 1.5 | 21.4 | 14.2 | 80.2 | 81.5 | 86.1 |
| MUC-2 | 100 | 23.8 | 26.0 | 72.1 | 89.1 | 86.8 |
| MUC-3 | 8.2 | 15.5 | 27.2 | 79.8 | 77.0 | 76.1 |
| Occludin | 43.9 | 7.7 | 32.0 | 86.2 | 82.9 | 91.0 |
| ZO-1 | 33.1 | 4.9 | 25.0 | 75.6 | 91.8 | 87.9 |
| TFF-3 | 41.0 | 27.6 | 25.6 | 74.1 | 76.1 | 76.6 |
| Villin | 63.2 | 22.4 | 64.6 | 74.3 | 71.5 | 77.0 |
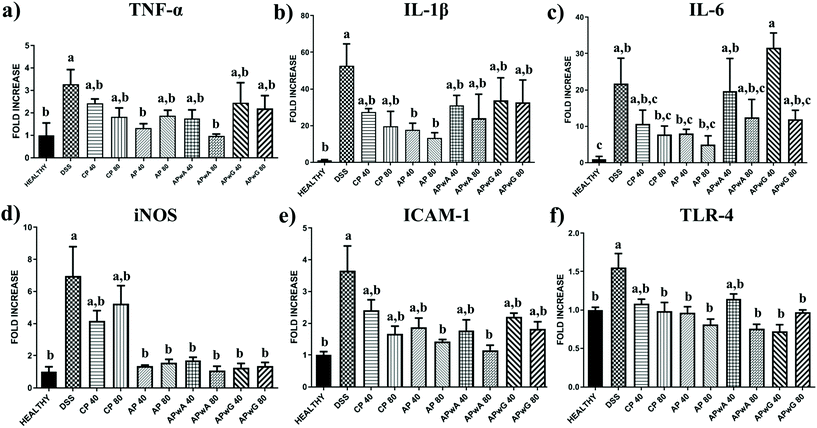
As expected, DSS-induced colitic inflammation was characterised by an altered immune response, which was evidenced by an increasing mRNA expression of the pro-inflammatory cytokines in comparison with non-colitic mice (Fig. 4). TNF-α expression decreased in colitis mice treated with original or modified pectin although this reduction was only statistically significant (p < 0.05) in those groups treated with original AP and APwA pectin at doses of 40 and 80 mg kg−1, respectively, showing most similar values to those of the healthy group. No significant differences were observed between APwG fractions at both doses. ANN analysis corroborated these results; TNF-α was one of the most relevant parameters in the profiles of mice treated with APwA, showing a higher reduction compared to other mice groups (Table 3). In addition, TNF-α was especially relevant to discriminate the profiles of the DSS control by ANN, indicating that this marker showed an accentuated decrease in experimental mice groups treated with most pectin samples. Similarly, IL-1β was significantly reduced when AP was administered at 40 and 80 mg kg−1 while no significant differences were observed among the rest of the groups. IL-6 levels in CP and AP treatments at the two studied doses (40 and 80 mg kg−1) were reduced, although differences between groups were not statistically relevant due to the biological variability. It should be noted that APwA and APwG did not reduce the expression of IL-6 at 40 mg kg−1, showing similar values to those of the DSS group, indicating a loss of potential immunostimulatory activity. IL-6 was also the most relevant parameter in ANN profiles for the CP control, original AP and APwG (Table 3). The ANN corroborates that CP and original AP treatments reduced this pro-inflammatory cytokine. In the case of APwG, analysis of variable importance indicates that the negative results obtained for this type of pectin did not exert any relevant effect on IL-6 levels.
Remarkably, iNOS expression was significantly decreased in all groups treated with AP and modified artichoke pectin fractions at both doses, reaching expression levels similar to those of the healthy group. ANN analysis revealed that iNOS was an important parameter to discriminate the profiles of mice treated with CP (Table 3) because this was the only treatment that did not result in a reduction of iNOS levels. This fact may be attributed to structural differences found between the two pectins studied; thus CP presented a higher DM (71%) than AP (19.5%) and modified artichoke pectins (24%) (ESI Table S2†).
Another pro-inflammatory marker determined was the transmembrane receptor involved in the inflammatory process ICAM-I (Fig. 4e), which was significantly reduced when AP and APwA were administered at doses of 80 mg kg−1, reaching values similar to those of the healthy control. ANN analysis showed that ICAM-I was the most relevant marker to discriminate the biochemical profiles of DSS control groups because all pectin and modified pectin treatments led to a decrease in the levels of this parameter (Table 3). ICAM-I was also the most important parameter in the profiles of mice treated with APwA pectin as it was drastically reduced. Interestingly, ICAM-I was relevant in APwG groups because this treatment led to a lower reduction in the levels of ICAM-I compared to other pectin samples.
A decrease in TLR-4 (Fig. 4f) expression was observed when pectin or modified pectin was administered to mice groups being significant under all conditions except for CP and APwA treatments at 40 mg kg−1. ANN indicated that TLR-4 levels differentiated the DSS control from the rest of the mice groups (Table 3) and corroborated the overall anti-inflammatory effect in the expression of TLR-4 produced by all pectin and modified samples.
In general, a reduction of all pro-inflammatory markers is produced, although the differences are not significant in several cases. ANN models confirmed the potential activity of AP and APwA fractions to modulate cytokine expression.
On the other hand, a group of anti-inflammatory markers such as the expression of barrier intestinal proteins were studied (Fig. 5). MUC-1 expression increased in those groups treated with original AP. However, a great variability between individuals was observed. ANN analysis showed that MUC-1 was an important factor in the profiles of original AP treated groups (Table 3) as it produced a relevant increase in the levels of this protein, showing the highest expression values.
In contrast, MUC-2 (Fig. 5b) levels were drastically low in all studied groups with respect to the healthy control, showing values similar to those of DSS control groups, so no positive effect on this marker was observed. Therefore, this was the most relevant marker to discriminate the profiles of the healthy mice group by ANN (Table 3). Similarly, the expression of MUC-3 (Fig. 5c) for all tested groups was significantly lower than that in the healthy group, with the exception of original AP and APwA treatments at 80 and 40 mg kg−1, respectively.
With regard to other membrane proteins determined, Occludin (Fig. 5d) levels were only significantly high in the group treated with AP at 80 mg kg−1. ANN patterns indicated that Occludin was a relevant parameter in the healthy mice group and those mice groups treated with original AP (Table 3), as no relevant increases were observed in the levels of Occludin when other pectin samples were administered. Moreover, this parameter was also relevant to discriminate mice groups treated with APwG highlighting the absence of any positive effect; these groups showed the lowest expression of this barrier protein compared to other pectin treatments.
In addition, no relevant differences were observed in TFF-3 levels (Fig. 5f) between different pectin treatments due to the high variability between individuals. The expressions of ZO-1 and Villin (Fig. 5e and g) were increased only for CP treatments at 40 mg kg−1 and no significant differences were observed between modified pectin fractions and original AP. These results were corroborated by ANN analysis, revealing that Villin was an important parameter in the healthy mice group and mice treated with CP (Table 3). This fact indicates that treatment of mice groups with CP was one of the most effective ones for reducing inflammatory cytokine IL-6 (as explained above) leading to an increase in the expression of barrier protein Villin. In contrast, ZO-1 was a relevant marker in the biochemical profiles of mice treated with APwG.
As explained, some of these differences were not statistically significant due to the high interindividual variability. However, ANN modelling corroborates differences observed in the obtained results and allows determining the most relevant biochemical markers to be considered in each mice group. Therefore, a dose-dependent response was observed for several biochemical markers: pro-inflammatory TNF-α and IL-1β, intestinal proteins and receptors MUC-1, Occludin and TLR-4. AP was especially efficient to reduce the expression of pro-inflammatory cytokine IL-6 compared to other samples, while APwA produced the most relevant effect on the pro-inflammatory receptor ICAM-I decrease. However, galactose-free modification resulted in a loss of pro-inflammatory cytokine regulation. In general, for APwG, the variable importance analysis highlights the negative results obtained for this type of modified pectin, so the ANN model determined higher levels of inflammatory markers ICAM-I and IL-6 and lower expression of intestinal barrier proteins Occludin and ZO-1 in the profiles of these groups compared to the rest of the treatments. Therefore, enzymatic modification with β-galactosidase from B. circulans produced a significant loss of pectin bioactivity.
4. Discussion
The current work presents a study of the in vivo intestinal anti-inflammatory effect of AP and modified AP fractions in a DSS model of mice colitis. The acute inflammatory response involves high expression of macrophage-derived cytokine profiles with a high participation of TNF-α, IL-1β and iNOS as well as adhesion molecule ICAM-I that enhances leukocyte endothelial transmigration, leading to tissue damage.6 Several studies reported in vitro and in vivo bioactivity of pectin and pectic substances highlighting the importance of specific structural features. One of the most relevant characteristics of AP is the high presence of arabinose in its ramified domains and low DM. According to previous studies, arabinose and galactose contents exert an important influence on pectin anti-inflammatory activity. Zhang et al.23 reported that pectic substances with high arabinose contents inhibit the production of IL-6 and TNF-α in LPS-stimulated RAW264.7 cells while a pectic polysaccharide from alfalfa, consisting mainly in the RG-I domain of L-arabinosyl and D-galactosyl units, showed potential inhibition of IL-1β and IL-6 gene expressions in the same cell line.24 Arabinogalactan from edible jambo fruits mainly composed of galactose and arabinose attenuated the pro-inflammatory secretion induced by an inflammatory agent in THP-1 cells,25 and silver linden flower pectins with RG-I domains rich in arabinogalactans suppressed iNOS expression and showed macrophage-stimulating activities,26 indicating that neutral sugar content of pectin determines its anti-inflammatory activity. However, the higher influence of galactose than arabinose was not reported in previous studies. In our study, galactose content in AP proved to be especially relevant to preserve pectin bioactivity (i.e. when galactose was removed in APwG modified pectin, this product did exert little relevant anti-inflammatory effect) while APwA maintained an important/significant pectin anti-inflammatory activity.The DM also exerts a great influence on pectin bioactivity according to previous in vitro studies. In this sense, in THP-1 macrophages treated with highly methyl-esterified HG branched by arabinogalactans and arabinans, TNF-α and IL1-β secretion was reduced in the presence of a pro-inflammatory agent.14 Similar in vitro immunostimulatory properties of sweet pepper pectin were also preserved in modified fractions obtained by acid hydrolysis where side chains had been removed showing lower MW and DM.27 In addition, lavender pectic polysaccharides containing a low-acetylated and high-methoxylated HG domain and RG-I fragments rich in arabinan and arabinogalactan showed anti-inflammatory activity on murine macrophages,28 and smaller pectic oligosaccharide chains may also regulate anti-inflammatory cytokine secretion (IL-1 receptor antagonist and IL-10) and may also inhibit the activity of IL-1β.29 Selenylation of low methyl-esterified pectin from Ulmus pumila L., containing galactan and glucan in its side chains, inhibited nitric oxide production in RAW 264.7 cells resulting in a potential anti-inflammatory activity.30 These in vitro studies indicate that low methyl-esterified pectin may exert anti-inflammatory activity, similar to high methyl-esterified pectin or even higher. These results agree with our study, where low methyl-esterified AP showed anti-inflammatory potential, which was even higher than the one of high methyl-esterified CP with recognised activity as the control.
Several studies reported in vivo anti-inflammatory properties of CP. Faecal microbiota transplantation combined with pectin in DSS-induced colitis mice enhanced its positive effects on the colonic ratio and DAI and reduced the expression of TNF-α, IL-1β and IL-6.31 A reduction in IL-1β, IL-6, and TNF-α in the murine model of endotoxin shock has also been reported for CP treatment, as well as a decrease in IL-6 secretion from Toll-like receptor-activated RAW264.7 pretreated with CP. However, when this study was performed using polygalacturonic acid, treatment was not effective, highlighting the importance of neutral sugars present in side chains of pectin.32 Pacheco et al.6 studied the expression of the inflammatory cytokine panel in the DSS-model of mice colitis, which was reduced after the administration of CP and orange by-products. These results agree with our study where a similar reduction in pro-inflammatory cytokine expression was observed for AP pectin and APwA, corroborating its immunostimulatory potential. It should be noted that these two samples as well as CP used as the positive control show high galactose contents.
AP samples evaluated in our study may interact with the TLR-4 receptor leading to an overall decrease in the expression of TNF-α, IL-1β and IL-6 biomarkers. This mechanism of action was previously suggested by Liu et al.33 who evaluated the protective efficacy on intestinal toxicities and carcinogenesis of an apple oligogalactan by targeting the LPS/TLR-4/NF-κB pathway in DSS-treated mice. These authors reported a decrease in the expression of pro-inflammatory cytokines. On the other hand, it has been demonstrated that TLR signalling is suppressed by MUC-1,34 so AP enhances MUC-1 expression leading to a decrease in TLR-4 levels and therefore, low expression of pro-inflammatory cytokines IL-1, IL-6 and TNF-α. This mechanism may explain some of the results obtained in the present study.
Another interesting mechanism that may contribute to interpretation of the anti-inflammatory effects of AP is the inhibition of carbohydrate-binding protein galectin-3. Previous studies using CP found that low Mw modified fractions reduced inflammation, fibrosis formation in organs and tissues and cancer progression in a mouse model of colitis-associated colon cancer by inhibiting galectin-3 to its ligand. This process may induce apoptosis of cancerous cells.35,36 In our study, AP modification did not enhance its bioactivity although it should be noted that Mw distribution patterns of APwG were similar to unmodified pectin. On the other hand, an in vivo study of anti-inflammatory properties of apple pectin showed a decrease of TLR-4 and TNF-α levels in illeal tissue of diet-induced obese rats,37 and apple pectin fractions rich in galactose reduced tumour development in a mice model of colitis-associated colon cancer through the inhibition of galectin-3.36 These results agree with those of our study where a significant reduction in galactose content present in pectin chains led to a dramatic loss of its anti-inflammatory activity, so galactose content of AP may be correlated with pectin capacity to inhibit galectin-3.
In vivo studies dealing with alternative sources of pectin found that RG-I fractions from potato pectin reduce the proliferation of DLD1 and HCT116 colon cancer cells in a dose- and time-dependent manner, as well as ICAM-I expression. The presence of linear GalA segments as well as neutral sugar side chains enhanced the bioactivity of these extracts.38 However, in our study it was found that higher linearity and lower extent of branching of modified pectin fractions did not result in an increase of their potential bioactivity, although APwA (which showed higher linearity and lower extent of branching as a consequence of enzymatic modification) preserved its anti-inflammatory properties. On the other hand, noni fruit polysaccharides, containing RG-I regions with high neutral sugar contents, such as arabinogalactans and arabinans, reduced the DAI and promoted the expression of mucosal and tight junction proteins like Occludin and ZO-1 in DSS-induced IBD mice.5 Mzoughi et al.39 reported that a pectic polysaccharide from Suaeda fruticosa, rich in arabinose and galactose, exerted an anti-inflammatory effect in rats at doses of 100 mg per kg body weight. Modified pectic polysaccharides from turmeric showing a high neutral sugar content, especially galactose and rhamnose, and low Mw (13 kDa) reduced ulcer in rats by decreasing pro-inflammatory factors like TNF-α and galectin-3 levels.40 These studies indicate that a high neutral sugar content and the degree of branching of pectin enhance its anti-inflammatory potential.
However, in our study the galactose content of pectins (ESI Table S2†) cannot explain differences observed between anti-inflammatory effects of CP and AP. These differences can also be due to low DM of AP. As has previously been indicated, DM of pectin is one crucial factor affecting its anti-inflammatory potential. Low methyl-esterified pectin from Opuntia microdasys cladodes exerted an anti-inflammatory effect in mice and reduced gastric ulcer in rats at doses of 100 mg per kg body weight.41 Similarly, low methyl-esterified pectic substances isolated from common pondweed showed anti-endotoxemic activity in mice by decreasing the expression of TNF-α and IL-1β and increasing the levels of anti-inflammatory cytokines such as IL-10.42 These studies agree with our results, where low methyl-esterified AP showed a higher immunomodulatory capacity than high methyl-esterified CP used as the control. Considering the results obtained in our work, low methyl-esterified pectins like AP may be proposed as good candidates for their anti-inflammatory properties.
Immunostimulatory effects in mice of both low and high methyl-esterified CP have been compared, showing that low methyl-esterified pectin decreased TNF-α release and increased production of the anti-inflammatory cytokine IL-10 while high methyl-esterified pectin had no effect on cytokine production.43 Sahasrabudhe et al.44 demonstrated that low methyl-esterified pectin inhibits TLR-2 and specifically inhibits the pro-inflammatory TLR-2–TLR-1 pathway by interacting with TLR-2 through electrostatic forces between non-esterified GalA and positive charges on the TLR-2 ectodomain. This mechanism of action may explain differences observed between high and low methyl-esterified pectins obtained in our study, so AP may show a higher inhibition of Toll like receptors pathways than CP due to its lower DM.
As explained, a great variability was observed in MUC-1 and TLR-4 levels in those groups treated with modified AP. Differences between groups were very subtle and biochemical profiles were compared through ANN modelling indicating that galactose content significantly contributes to pectin bioactivity. Argüeso et al.15 found that modified citrus pectin induces MUC-1. However, this positive effect was reported to be highly dependent on galactose content of pectin whereas APwG yielded higher MUC-1 expression than APwA in our study.
5. Conclusions
This work addresses the influence of structural characteristics of artichoke pectin and modified pectin fractions on in vivo anti-inflammatory activity. Original artichoke pectin gave rise to a lower expression of pro-inflammatory cytokines in a dose-dependent manner and a higher expression of some intestinal barrier proteins. To establish characteristic expression profiles of biochemical parameters for each type of treatment, ANN models were developed. It has been demonstrated that DM exerts a great influence together with pectin neutral sugar content, degree of branching and Mw. With regard to pectin monomeric composition, it has been observed that galactose content is especially relevant to preserve pectin anti-inflammatory properties while arabinose removal does not result in a drastic loss of bioactivity. Further studies are needed to completely elucidate the complex mechanism of action of pectin, based on its structural features. Taking into account these results, AP can be used as a functional ingredient that may be extracted from artichoke industrial by-products as novel pectin source.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work has been funded by MICINN of Spain, Projects AGL2014-53445-R and AGL2017-84614-C2-1-R. Carlos Sabater thanks his FPU Predoc contract from Spanish MECD (FPU14/03619).References
- L. N. Gerschenson, The production of galacturonic acid enriched fractions and their functionality, Food Hydrocolloids, 2017, 68, 23–30 CrossRef CAS.
- S. E. Broxterman, P. Picouet and H. A. Schols, Acetylated pectins in raw and heat processed carrots, Carbohydr. Polym., 2017, 177, 58–66 CrossRef CAS PubMed.
- M. A. Atmodjo, Z. Hao and D. Mohnen, Evolving views of pectin biosynthesis, Annu. Rev. Plant Biol., 2013, 64, 747–779 CrossRef CAS PubMed.
- M. Marić, A. N. Grassino, Z. Zhu, F. J. Barba, M. Brnčić and S. R. Brnčić, An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction, Trends Food Sci. Technol., 2018, 76, 28–37 CrossRef.
- M. Jin, Y. Wang, X. Yang, H. Yin, S. Nie and X. Wu, Structure characterization of a polysaccharide extracted from noni (Morinda citrifolia L.) and its protective effect against DSS-induced bowel disease in mice, Food Hydrocolloids, 2019, 90, 189–197 CrossRef CAS.
- M. T. Pacheco, T. Vezza, P. Diez-Echave, P. Utrilla, M. Villamiel and F. J. Moreno, Anti-inflammatory bowel effect of industrial orange by-products in DSS-treated mice, Food Funct., 2018, 9, 4888–4896 RSC.
- G. Zhu, H. Wang, T. Wang and F. Shi, Ginsenoside Rg1 attenuates the inflammatory response in DSS-induced mice colitis, Int. Immunopharmacol., 2017, 50, 1–5 CrossRef CAS PubMed.
- A. L. M. Silveira, A. V. M. Ferreira, M. C. de Oliveira, M. A. Rachid, L. F. da Cunha Sousa, F. dos Santos Martins, A. C. Gomes-Santos, A. T. Vieira and M. M. Teixeira, Preventive rather than therapeutic treatment with high fiber diet attenuates clinical and inflammatory markers of acute and chronic DSS-induced colitis in mice, Eur. J. Nutr., 2017, 56, 179–191 CrossRef CAS PubMed.
- M. Kim, L. Friesen, J. Park, H. M. Kim and C. H. Kim, Microbial metabolites, short-chain fatty acids, restrain tissue bacterial load, chronic inflammation, and associated cancer in the colon of mice, Eur. J. Immunol., 2018, 48, 1235–1247 CrossRef CAS PubMed.
- Y. R. Song, S. K. Sung, M. Jang, T. G. Lim, C. W. Cho, C. J. Han and H. D. Hong, Enzyme-assisted extraction, chemical characteristics, and immunostimulatory activity of polysaccharides from Korean ginseng (Panax ginseng Meyer), Int. J. Biol. Macromol., 2018, 116, 1089–1097 CrossRef CAS PubMed.
- K. Y. Abboud, B. B. da Luz, J. L. Dallazen, M. F. de Paula Werner, C. B. B. Cazarin, M. R. M. Junior, M. Iacomini and L. M. Cordeiro, Gastroprotective effect of soluble dietary fibres from yellow passion fruit (Passiflora edulis f. flavicarpa) peel against ethanol-induced ulcer in rats, J. Funct. Foods, 2019, 54, 552–558 CrossRef CAS.
- C. L. Leivas, L. F. Nascimento, W. M. Barros, A. R. Santos, M. Iacomini and L. M. Cordeiro, Substituted galacturonan from starfruit: Chemical structure and antinociceptive and anti-inflammatory effects, Int. J. Biol. Macromol., 2016, 84, 295–300 CrossRef CAS PubMed.
- Y. Sun, L. Fan, W. Mian, F. Zhang, X. Liu, Y. Tang, X. Zeng, Q. Mei and Y. Li, Modified apple polysaccharide influences MUC-1 expression to prevent ICR mice from colitis-associated carcinogenesis, Int. J. Biol. Macromol., 2018, 120, 1387–1395 CrossRef CAS PubMed.
- A. F. de Oliveira, G. E. do Nascimento, M. Iacomini, L. M. C. Cordeiro and T. R. Cipriani, Chemical structure and anti-inflammatory effect of polysaccharides obtained from infusion of Sedum dendroideum leaves, Int. J. Biol. Macromol., 2017, 105, 940–946 CrossRef PubMed.
- P. Argüeso, A. Guzman-Aranguez, F. Mantelli, Z. Cao, J. Ricciuto and N. Panjwani, Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier, J. Biol. Chem., 2009, 284, 23037–23045 CrossRef PubMed.
- C. Sabater, N. Corzo, A. Olano and A. Montilla, Enzymatic extraction of pectin from artichoke (Cynara scolymus L.) by-products using Celluclast® 1.5 L, Carbohydr. Polym., 2018, 190, 43–49 CrossRef CAS PubMed.
- C. Sabater, V. Sabater, A. Olano, A. Montilla and N. Corzo, Ultrasound-assisted extraction of pectin from artichoke by-products. An artificial neural network approach to pectin characterisation, Food Hydrocolloids, 2020, 98, 105238 CrossRef CAS.
- C. Sabater, A. Olano, N. Corzo and A. Montilla, GC–MS characterisation of novel artichoke (Cynara scolymus) pectic-oligosaccharides mixtures by the application of machine learning algorithms and competitive fragmentation modelling, Carbohydr. Polym., 2019, 205, 513–523 CrossRef CAS PubMed.
- C. Sabater, A. Ferreira-Lazarte, A. Montilla and N. Corzo, Enzymatic Production and Characterization of Pectic Oligosaccharides Derived from Citrus and Apple Pectins: A GC-MS Study Using Random Forests and Association Rule Learning, J. Agric. Food Chem., 2019, 67, 7435–7447 CrossRef CAS PubMed.
- P. Olivera, S. Danese, N. Jay, G. Natoli and L. Peyrin-Biroulet, Big data in IBD: a look into the future, Nat. Rev. Gastroenterol. Hepatol., 2019, 16, 312–321 CrossRef PubMed.
- C. Bergmeir and J. M. Benitez, Neural Networks in R Using the Stuttgart Neural Network Simulator: RSNNS, J. Stat. Softw., 2012, 46, 1–26 Search PubMed , URL http://www.jstatsoft.org/v46/i07/ (accessed 26/08/2019).
- E. LeDell, N. Gill, S. Aiello, A. Fu, A. Candel, C. Click, T. Kraljevic, T. Nykodym, P. Aboyoun, M. Kurka and M. Malohlava, h2o: R Interface for ‘H2O’. R package version 3.22.1.1, 2019, URL https://CRAN.R-project.org/package=h2o (accessed 26/08/2019) Search PubMed.
- Y. Zhang, X. Pan, S. Ran and K. Wang, Purification, structural elucidation and anti-inflammatory activity in vitro of polysaccharides from Smilax china L., Int. J. Biol. Macromol., 2019, 139, 233–243 CrossRef CAS PubMed.
- L. Chen, J. Liu, Y. Zhang, B. Dai, Y. An and L. Yu, Structural, thermal, and anti-inflammatory properties of a novel pectic polysaccharide from alfalfa (Medicago sativa L.) stem, J. Agric. Food Chem., 2015, 63, 3219–3228 CrossRef CAS PubMed.
- C. S. Tamiello, G. E. do Nascimento, M. Iacomini and L. M. Cordeiro, Arabinogalactan from edible jambo fruit induces different responses on cytokine secretion by THP-1 macrophages in the absence and presence of proinflammatory stimulus, Int. J. Biol. Macromol., 2018, 107, 35–41 CrossRef CAS PubMed.
- Y. N. Georgiev, B. S. Paulsen, H. Kiyohara, M. Ciz, M. H. Ognyanov, O. Vasicek, F. Rise, P. N. Denev, A. Lojek, T. G. Batsalova, B. M. Dzhambazov, H. Yamada, R. Lund, H. Barsett, A. I. Krastanov, I. Z. Yanakieva and M. G. Kratchanova, Tilia tomentosa pectins exhibit dual mode of action on phagocytes as β-glucuronic acid monomers are abundant in their rhamnogalacturonans I, Carbohydr. Polym., 2017, 175, 178–191 CrossRef CAS PubMed.
- G. E. do Nascimento, S. M. B. Winnischofer, M. I. Ramirez, M. Iacomini and L. M. C. Cordeiro, The influence of sweet pepper pectin structural characteristics on cytokine secretion by THP-1 macrophages, Food Res. Int., 2017, 102, 588–594 CrossRef CAS PubMed.
- Y. N. Georgiev, B. S. Paulsen, H. Kiyohara, M. Ciz, M. H. Ognyanov, O. Vasicek, F. Rise, P. N. Denev, H. Yamada, A. Lojek, V. Kussovski, H. Barsett, A. I. Krastanov, I. Z. Yanakieva and M. G. Kratchanova, The common lavender (Lavandula angustifolia Mill.) pectic polysaccharides modulate phagocytic leukocytes and intestinal Peyer's patch cells, Carbohydr. Polym., 2017, 174, 948–959 CrossRef CAS PubMed.
- H. Tan, W. Chen, Q. Liu, G. Yang and K. Li, Pectin Oligosaccharides Ameliorate Colon Cancer by Regulating Oxidative Stress-and Inflammation-Activated Signaling Pathways, Front. Immunol., 2018, 9, 1504 CrossRef PubMed.
- J. H. Lee, Y. K. Lee, Y. R. Choi, J. Park, S. K. Jung and Y. H. Chang, The characterization, selenylation and anti-inflammatory activity of pectic polysaccharides extracted from Ulmus pumila L., Int. J. Biol. Macromol., 2018, 111, 311–318 CrossRef CAS PubMed.
- Q. Hu, M. H. Lü and M. M. Deng, Effects of Fecal Microbiota Transplantation on DSS-Induced Colitis Mice May Be Partly Owing to Enhanced Polarization of Macrophage M2, Gastroenterology, 2018, 154, S-33 CrossRef.
- K. Ishisono, T. Yabe and K. Kitaguchi, Citrus pectin attenuates endotoxin shock via suppression of Toll-like receptor signaling in Peyer's patch myeloid cells, J. Nutr. Biochem., 2017, 50, 38–45 CrossRef CAS PubMed.
- L. Liu, Y. H. Li, Y. B. Niu, Y. Sun, Z. J. Guo, Q. Li, C. Li, J. Feng, S. Cao and Q. B. Mei, An apple oligogalactan prevents against inflammation and carcinogenesis by targeting LPS/TLR4/NF-κB pathway in a mouse model of colitis-associated colon cancer, Carcinogenesis, 2010, 31, 1822–1832 CrossRef CAS PubMed.
- K. Ueno, T. Koga, K. Kato, D. T. Golenbock, S. J. Gendler, H. Kai and K. C. Kim, MUC1 mucin is a negative regulator of toll-like receptor signalling, Am. J. Respir. Cell Mol. Biol., 2008, 38, 263–268 CrossRef CAS PubMed.
- I. Eliaz, U.S. Patent9427449, U.S. Patent and Trademark Office, Washington, DC, 2016, URL https://patents.google.com/patent/US9427449B2/en (accessed 12/09/2019) Search PubMed.
- Y. Li, L. Liu, Y. Niu, J. Feng, Y. Sun, X. Kong, Y. Chen, X. Chen, H. Gan, S. Cao and Q. Mei, Modified apple polysaccharide prevents against tumorigenesis in a mouse model of colitis-associated colon cancer: role of galectin-3 and apoptosis in cancer prevention, Eur. J. Nutr., 2012, 51, 107–117 CrossRef CAS PubMed.
- T. Jiang, X. Gao, C. Wu, F. Tian, Q. Lei, J. Bi, B. Xie, H. Y. Wang, S. Chen and X. Wang, Apple-derived pectin modulates gut microbiota, improves gut barrier function, and attenuates metabolic endotoxemia in rats with diet-induced obesity, Nutrients, 2016, 8, 126 CrossRef PubMed.
- E. G. Maxwell, I. J. Colquhoun, H. K. Chau, A. T. Hotchkiss, K. W. Waldron, V. J. Morris and N. J. Belshaw, Rhamnogalacturonan I containing homogalacturonan inhibits colon cancer cell proliferation by decreasing ICAM1 expression, Carbohydr. Polym., 2015, 132, 546–553 CrossRef CAS PubMed.
- Z. Mzoughi, A. Abdelhamid, C. Rihouey, D. Le Cerf, A. Bouraoui and H. Majdoub, Optimized extraction of pectin-like polysaccharide from Suaeda fruticosa leaves: Characterization, antioxidant, anti-inflammatory and analgesic activities, Carbohydr. Polym., 2018, 185, 127–137 CrossRef CAS PubMed.
- H. M. Rajagopal, S. B. Manjegowda, C. Serkad and S. M. A. Dharmesh, Modified pectic polysaccharide from turmeric (Curcuma longa) with antiulcer effects via anti–secretary, mucoprotective and IL–10 mediated anti–inflammatory mechanisms, Int. J. Biol. Macromol., 2018, 118, 864–880 CrossRef CAS PubMed.
- M. Jouini, A. Abdelhamid, M. A. Chaouch, D. le Cerf, A. Bouraoui, H. Majdoub and H. B. Jannet, Physico-chemical characterization and pharmacological activities of polysaccharides from Opuntia microdasys var. rufida cladodes, Int. J. Biol. Macromol., 2018, 107, 1330–1338 CrossRef CAS PubMed.
- S. V. Popov, G. Y. Popova, N. M. Paderin, O. A. Koval, R. G. Ovodova and Y. S. Ovodov, Preventative antiinflammatory effect of potamogetonan, a pectin from the common pondweed Potamogeton natans L., Phytother. Res., 2007, 21, 609–614 CrossRef CAS PubMed.
- S. V. Popov, P. A. Markov, G. Y. Popova, I. R. Nikitina, L. Efimova and Y. S. Ovodov, Anti-inflammatory activity of low and high methoxylated citrus pectins, Biomed. Prev. Nutr., 2013, 3, 59–63 CrossRef.
- N. M. Sahasrabudhe, M. Beukema, L. Tian, B. Troost, J. Scholte, E. Bruininx, G. Bruggeman, M. van der Berg, A. Scheurink, H. A. Schols, M. M. Faas and P. de Vos, Dietary fiber pectin directly blocks Toll-like receptor 2–1 and prevents doxorubicin-induced ileitis, Front. Immunol., 2018, 9, 383 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9fo02221j |
| This journal is © The Royal Society of Chemistry 2019 |