Open Access Article
Open Access ArticleCharacterisation of peroxidation products arising from culinary oils exposed to continuous and discontinuous thermal degradation processes†
Adam
Le Gresley
 *a,
Gilbert
Ampem
a,
Martin
Grootveld
b,
Benita C.
Percival
b and
Declan P.
Naughton
a
*a,
Gilbert
Ampem
a,
Martin
Grootveld
b,
Benita C.
Percival
b and
Declan P.
Naughton
a
aDepartment of Chemistry and Pharmaceutical Sciences, SEC Faculty, Kingston University, Kingston-upon-Thames, Surrey KT1 2EE, UK. E-mail: a.legresley@kingston.ac.uk; Tel: +44 (0)20 84177432
bHealth and Life Sciences, De Montfort University, Leicester, LE1 9BH, UK
First published on 19th November 2019
Abstract
High-resolution NMR analysis has been used, for the first time, to identify, putatively, two new secondary aldehydic lipid oxidation products in culinary oils. The impact of heating and cooling times on the thermal stability, fatty acid composition and lipid oxidation product (LOP) concentrations have been analysed for continuous and discontinuous heating periods (180 °C). The susceptibility of the selected oils to thermal oxidation for the different heating episodes has been evaluated via the detection and determination of LOPs, particularly cytotoxic and genotoxic aldehydes. The identities and quantities of these LOPs evolved throughout a 2.0 hour period. Results acquired indicated that sunflower oil was more resistant to discontinuous oxidation than rapeseed and olive oils, however overall discontinuous heating resulted in more LOPs.
1. Introduction
1.1 Rationale
The reported health benefits of Unsaturated Fatty Acid (UFA)-rich culinary oils have resulted in an increase in their popularity and use.1,2 There exists, however, substantial evidence published over the past vicennial that UFA-rich culinary oils (particularly highly peroxidatively-susceptible PUFA-laden ones) stored for long periods or subjected to any form of heating may pose health hazards to consumers.3 As a common practice, the repeated use of reheated culinary oils at standard frying temperatures (180 °C) and beyond predisposes them to adverse thermo-oxidation, hydrolysis and polymerization processes.4 In spite of this, little concern is given to this potentially major problem owing to the focus largely falling on the putative atherogenic and carcinogenic properties of trans-fatty acids found in culinary oil/fat products and frying media.5We report herein a study involving commercially-available culinary oils and how their chemical compositions are influenced by their exposure to thermal stressing episodes when applied over both continuous and discontinuous timeframes. Particular attention is paid to secondary aldehydic LOPs. In this report, we address the following relationships regarding the evolution of LOPs during such thermal stressing periods conducted according to standard frying practices, specifically:
• The relationship between unsaturation status and susceptibility to thermal oxidation;
• The relationship between oil type and the identity of the LOPs evolved during thermal oxidation processes;
• The quantity of LOPs generated according to the type of thermal oxidation process that culinary oils frequently undergo.
Through the identification and quantification of LOPs produced in different types of thermo-oxidation approaches, it may be possible to identify the best oils and/or methods of heating in order to reduce the concentrations and classes of LOPs generated. This has the potential to aid in the prevention of emerging public health issues in humans associated with the dietary ingestion and/or inhalation of LOPs.5
1.2 Chemical changes to fatty acid (FA) chain composition
During thermal oxidation, both major and minor acyl groups of culinary fats and oils are degraded at variable rates to produce new compounds.6It has already been established that the intensities of signals generated in the 1H NMR spectra (Fig. 1) is proportional to the product of the number of protons that give rise to them and the concentration(s) of the assigned molecule(s) featured, and quantification can be performed via comparisons to the intensity of a validated internal reference standard.6,7 The calculations involved in this process for the quantification of the FAs indicated in Table 1 are shown in ESI (Summary S1).† In view of established literature, 1H NMR analysis was selected as a non-destructive, quantitative technique, which is capable of identifying and quantifying components without the requirement for their prior separation.
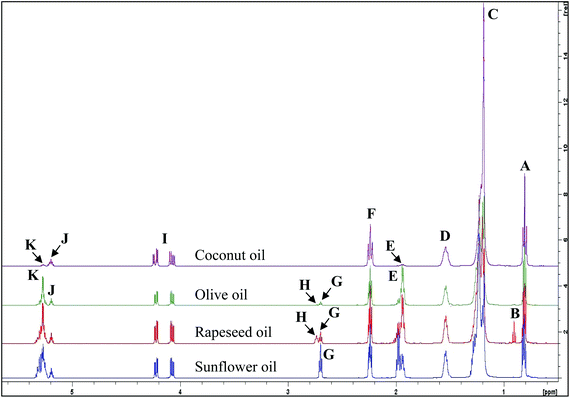 | ||
| Fig. 1 1H NMR spectra of major acylglycerol groups present in the 0.0–5.4 ppm regions of unheated culinary oils. The letter assignments of resonances correspond to those provided in Table 1. | ||
| Functional group | ||||
|---|---|---|---|---|
| Signal | Chemical shift (ppm) | Multiplicity | Condensed function | Classification |
| Abbreviations: d, doublet; t, triplet; m, multiplet; dd, double doublet; dt, double triplet; ω-3, omega-3 acyl groups; ω-6, omega-6 acyl groups; DHA, docosahexaenoyl acyl groups; EPA, eicosapentaenoyl acyl groups; ARA, arachidonoyl acyl groups. Letters assigned to signals correspond to those given in Fig. 1. | ||||
| A | 0.743–0.872 | t | –CH3 | Saturated, oleic and linoleic acyl groups |
| B | 0.872–0.936 | t | –CH3 | Unsaturated ω-3 acyl groups |
| C | 1.116–1.347 | t | –(CH2)n– | Acyl groups |
| D | 1.462–1.625 | m | –OCO–CH2–CH2– | Acyl groups except for DHA, EPA and ARA acyl groups |
| E | 1.859–2.056 | m | –CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– CH– |
Acyl groups except for –CH2– of DHA acyl group in β-position relative to the carbonyl function |
| F | 2.172–2.304 | dt | –OCO–CH2– | Acyl groups except for DHA acyl groups |
| G | 2.653–2.725 | t | ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) HC–CH2–CH HC–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) |
Diunsaturated ω-6 acyl groups |
| H | 2.725–2.782 | t | ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) HC–CH2–CH HC–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) |
Triunsaturated ω-3 acyl groups |
| I | 4.014–4.282 | dd, dd | –CH2OCOR | Glyceryl backbone groups |
| J | 5.157–5.229 | m | >CHOCOR | Glyceryl backbone groups |
| K | 5.229–5.368 | m | –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– CH– |
Acyl chain olefinic functions |
1.3 Oxidation products of FAs
As a consequence of the thermal degradation of unsaturated acyl groups in culinary oil samples, many LOPs of varying chemical reactivity have been shown to evolve,8 and all types of LOPs found in this study are listed in Tables 2 and 3, with their respective spectra shown in Fig. 2, 3, 4, 5 and 6.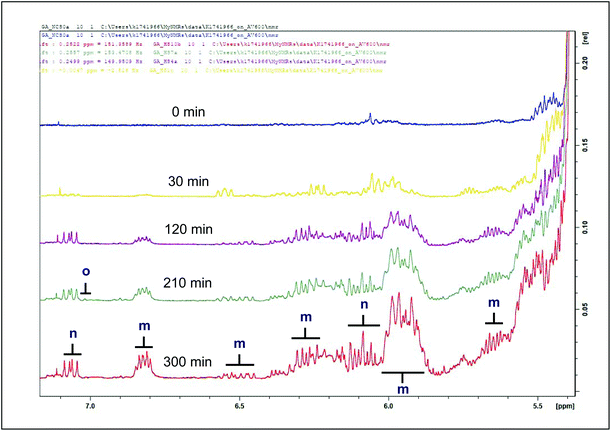 | ||
| Fig. 2 1H NMR spectra of conjugated diene hydroperoxydienes and hydroxymonoenes (primary LOPs), and olefinic resonances of α,β-unsaturated aldehydes present in the 5.4–7.1 ppm regions of sunflower oil thermally-stressed continuously throughout a 300 min period. For m and n there is overlap, suggesting several unsaturated chain lengths, hence the difference in apparent integral. Abbreviations: d, doublet; t, triplet; m, multiplet; dd, double doublet; CHPDs, conjugated hydroperoxydienes. Letter assignments correspond to those provided in Table 2. | ||
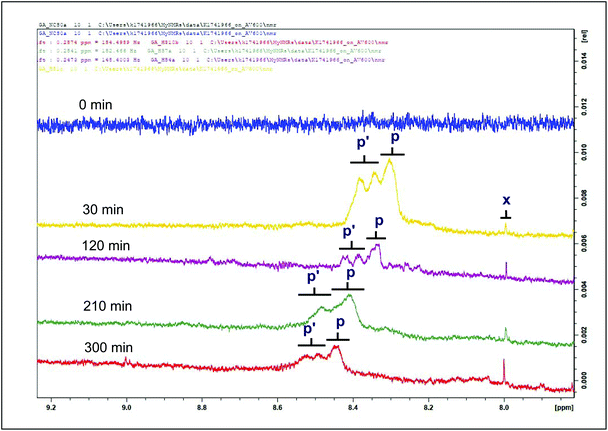 | ||
| Fig. 3 1H NMR spectra of a hydroperoxide group (primary LOPs) and methanoic acid present in the 7.5–9.2 ppm regions of sunflower oil thermally stressed continuously throughout a 300 min period. Signal p is a designated OOH– (E,E) hydroperoxide function. Signal p′ is a designated OOH– (Z,E) hydroperoxide function. Letter assignments correspond to those provided in Table 2. | ||
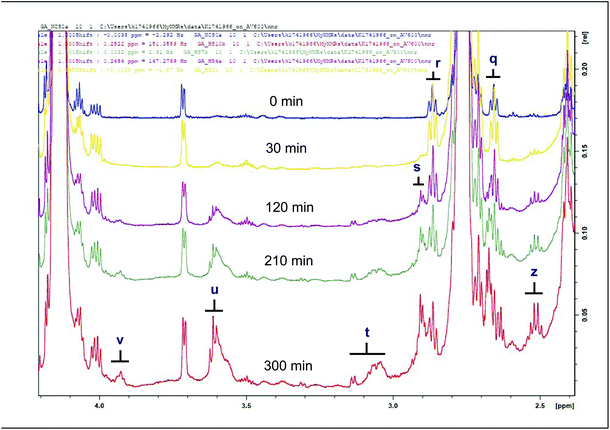 | ||
| Fig. 4 1H NMR spectra of epoxides (secondary LOPs) and primary alcohols present in the 2.4–4.2 ppm regions of sunflower oil thermally stressed continuously throughout a 300 min period. Letter assignments correspond to those in Table 3. | ||
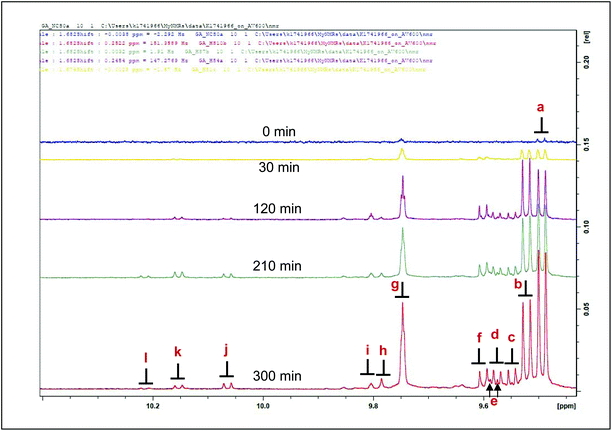 | ||
| Fig. 5 1H NMR spectra of aldehydes (secondary LOPs) present in the 9.3–10.4 ppm regions of sunflower oil thermally stressed continuously over a 300 min period. Letter assignments correspond to those in Table 4. | ||
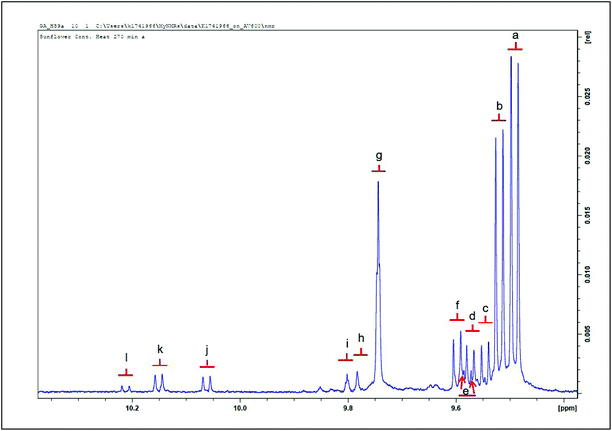 | ||
| Fig. 6 Expanded aldehydic –CHO function region (δ = 9.40–10.36 ppm) of 1H NMR spectra of a thermally-stressed sunflower oil. Letters assigned to signals correspond to those in Table 4. | ||
| Functional group | ||||
|---|---|---|---|---|
| Signal | Chemical shift (ppm) | Multiplicity | Condensed function | Classification |
| Abbreviations: s, singlet; d, doublet; t, triplet; m, multiplet; dd, double doublet; dt, double triplet. Letters assigned to signals correspond to those in Fig. 2 and 3. | ||||
| m | 5.456–5.544 | ddm | –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– CH– |
(E,E)-Conjugated olefinic protons of CHPDs (the C2 vinylic proton of (E)-2-alkenals is at 6.10 ppm, and the C3 one at 6.85 ppm). Olefinic resonances of (E,E)-alka-2,4-dienals are located at 6.04, 6.20 and 6.30 (ppm). Their C-3 olefinic proton has a multiplet signal at δ = 7.07 ppm. |
| m | 5.719–5.882 | ddm | –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– CH– |
|
| m | 6.094–6.182 | ddd | –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– CH– |
|
| m | 6.303–6.436 | ddtd | –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– CH– |
|
| m | 6.631–6.728 | ddm | –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– CH– |
|
| n | 6.888–6.957 | dddd | –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– CH– |
(Z,E)-Conjugated olefinic protons of CHPDs |
| n | 5.898–5.998 | ddm | –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– CH– |
|
| o | 6.849–6.888 | dd | –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– CH– |
(Z,E)-Conjugated olefinic protons of CHPDs |
| p/p′ | 8.200–9.200 | — | –OOH | CHPD and hydroperoxymonoene hydroperoxide functions |
| x | 7.667–7.681 | s | –COH | Methanoic acid |
| Functional group | ||||
|---|---|---|---|---|
| Signal | Chemical shift (ppm) | Multiplicity | Condensed function | Classification |
| Abbreviations: m, multiplet. Letter assignments correspond to those provided in Fig. 4. | ||||
| z | 2.128–2.182 | m | — | Unidentified |
| q | 2.258–2.335 | m | –CHOHC– | (E)-9,10-Epoxystearate |
| r | 2.488–2.532 | m | –CHOHC– | (Z)-9,10-Epoxystearate |
| s | 2.532–2.568 | m | –CHOHC–CHOHC– | 9,10–12,13-Diepoxyoctadecanoate |
| t | 2.651–2.796 | m | –CHOHC– | 9,10-Epoxy-octadecanoate; 9,10-Epoxy-12-octadecenoate (leukotoxin); and 12,13-Epoxy-9-octadecenoate (isoleukotoxin) |
| u | 3.172–3.303 | m | –CHOHC–CH2–CHOHC– | 9,10–12,13-Diepoxyoctadecanoate |
| v | 3.548–3.595 | m | α-CH2 | Primary alcohol LOPs |
The thermal degradation of unsaturated acyl groups in culinary oil yields the evolution of primary LOPs. Primary LOPs are unstable, short-lived intermediates whose detection is often difficult to recount.9 Their degradation further leads to the formation of secondary LOPs, which are designated stable species and as a result, do pose a greater health risk to living cells and organs. Primary LOPs detected in this study are listed in Table 2 and their respective spectra shown on Fig. 2 and 3, as well as in Fig. S1 and S2 (ESI†).
Throughout the 300 min heating duration, conjugated hydroperoxydienes and hydroxymonoenes, and olefinic resonances of α,β-UA, were observed to sequentially evolve with increasing heating time as shown in Fig. 2 for sunflower oil. Amongst the culinary oils, the primary LOPs were predominantly low or undetectable in coconut oil (Fig. S1†). The concentrations of these primary LOPs were found to be similar for monounsaturated fatty acid (MUFA)-rich olive and rapeseed oils. Nonetheless, higher signals were observed in thermally-stressed sunflower oil (Fig. S1†). The differences in the observable primary LOPs are ascribable to the differences in the UFA contents of the studied oils, i.e. sunflower oils has the highest content of particularly peroxidation-susceptible PUFAs, MUFA-rich oils (olive and rapeseed) are less so since MUFAs are much more resistant to peroxidation, and saturated fatty acids (present in coconut oil at levels of ca. 90% (w/w)) are virtually completely resistant to thermo-oxidation.
Hydroperoxides constitute primary LOPs, and their evolution with increasing heating time is shown in Fig. 3. A comparison of the two types of hydroperoxides amongst the studied culinary oils is shown in Fig. S2.† Whilst both (E,E)- and (Z,E)-hydroperoxides were detected in all oil types, their signal intensities increased as a function of heating time until 210 min, where a gradual disappearance of the intensity of the hydroperoxides groups was observed. This explains why olive and rapeseed oil spectra show a relatively flatter region in comparison to coconut and sunflower oil (Fig. S2†). This phenomenon was tracked in all studied oils with an illustration shown in Fig. 3. All these primary LOPs (conjugated hydroperoxydienes and hydroxymonoenes, and olefinic resonances of α,β-UA) have been reported to be present in sunflower oil (10 g thermally stressed placed in an oven for 540 min at 100 °C, and 4320 min at 70 °C on a 80 mm diameter × 15 mm Petri dish),9 and corn oils (retained at room temperature in closed receptacles for 121 min).10 Methanoic acid, which is the simplest carboxylic acid (which arises from the degradation of malondialdehyde, another aldehydic LOP), was also detected in the thermally-stressed oils (Fig. 3 and S2†). Being of low toxicity, methanoic acid is used as a food additive. The evolution of methanoic acid was proportional to the duration of thermal stressing at constant temperature, 180 °C (Fig. 3 and S2†).
Also classified as secondary LOPs, epoxides and primary alcohols were also detected in the studied oils (Table 3, and Fig. 4, S3†). These epoxides are proposed to be generated from linoleic and oleic acyl groups.6,10,12 The high level of distinction in the patterns of these products observed in the studied oils was ascribable to the identification of signals s and v, namely 9,10–12,13-diepoxyoctadecanoate (predominant in sunflower and rapeseed oils), and those of primary alcohols (predominant in coconut and sunflower oil) (Fig. S3†). Such fatty acid epoxides can cause degeneration and necrosis of leukocytes. They have also been associated with organ malfunction, breast carcinogenesis and cell proliferation.13,14 The presence of these LOPs in thermally-stressed culinary oils poses a significant health risk to consumers, and therefore such considerations should to be attended to. Epoxides have also been reported in thermo-oxidized sunflower and extra-virgin olive oils.10,12
The aldehydic LOPs discussed in this investigation can be categorised as either α,β-unsaturated (α,β-UAs) or saturated aldehydes.
Fig. 5 Shows the 1H NMR aldehydic-CHO function spectral regions demonstrating their evolution during the thermal stressing of culinary oil products.
α,β-Unsaturated aldehydes are considered more toxic than saturated ones.5,16,17 Appearing as doublets in the 1H NMR spectra acquired; signals a, b, c, d, e, f and j, classified as α,β-UAs; were identified as (E)-2-alkenals, (E,E)-2,4-alkadienals, 4,5-epoxy-(E)-alkenals, 4-hydroxy-(E)-2-alkenals, 4-hydroperoxy-(E)-2-alkenals, (Z,E)-2,4-alkadienals and (Z)-2-alkenals respectively.6,15 Meanwhile, there is a significant level of overlap of the hydroperoxy- and hydroxy-substituted (E)-2-alkenals. Signals g, h and i represent those arising from saturated aldehydes which are all triplets with small coupling constants. Signals g and i are assignable to n-alkanals, with the latter being representative of low-molecular-mass n-alkanals, predominantly n-propanal and n-butanal.6 Signal h is an oxygen-substituted n-alkanal class, i.e. 4-oxo-n-alkanals.6 Doublet signals k and l presumably represent alkenal species, probably (Z)-isomers from their corresponding J values (8.08 Hz).15
Pioneering studies in lipid oxidation process has made use of several classical qualitative and quantitative standard methods that made important contributions in understanding the mechanisms, dynamics and the evolution of both primary and secondary LOPs. However, these methods, which include peroxide value (PV), conjugated dienes (CDs) and conjugated trienes (CTs), para-anisidine value (pAV), and thiobarbituric acid-reactive substances (TBARS), have all been criticised as being laborious and lacking in specificity regarding the nature of potential LOPs conceivably analysed, giving rise to inaccurate interpretations and conclusions, and inefficient applications.18,19 Indeed, in addition to a marked lack of specificity, the still routinely employed TBARS assay is known to artefactually generate a variety of LOPs, both primary and secondary, during the heating stage (usually for a period of 15 min at ca. 95 °C).5 On the contrary, one-dimensional (1H) and two-dimensional (1H–1H and 1H–13C) nuclear magnetic resonance (NMR) analyses are established techniques that are faster and dependable with regard to the identification and determination of variable concentrations of cytotoxic and genotoxic LOPs in food lipid systems.5,6 The value of the NMR technique is further exemplified by the experimental applications of Pure Shift Yielded by Cherp Excitation (PSYCHE) and hyphenated diffusion techniques (PSYCHEiDOSY) for the resolution of small molecule resonances in complex multicomponent mixtures.20 Nonetheless, primary oxidation products experimentally determined as PV and CD values have been shown to positively correlated with lipid hydroperoxide levels determined by the 1H NMR technique.21
This research will therefore explore the of molecular ‘patterns’ of toxic LOPs generated in a wide range of culinary oil or fat products, particularly when exposed to thermal stressing episodes performed according to standard frying practices.
2. Materials and methods
2.1 Preparation of oil samples
Culinary oils were procured from a local supermarket outlet in Manchester, United Kingdom. These were: 100% organic virgin coconut oil (90.22% (w/w) SFAs, with unspecified amounts of MUFAs and PUFAs); olive oil (15.02% (w/w) SFAs, 76.38% (w/w) MUFAs, and 8.60% (w/w) PUFAs); rapeseed oil (7.96% (w/w) SFAs, 63.03% (w/w) MUFAs, and 29.01% (w/w) PUFAs); and sunflower oil (10.87% (w/w) SFAs, 28.26% (w/w) MUFAs, and 60.87% (w/w) PUFAs). Based on the percentage FA compositions originally printed on their labels (values in parenthesis) as purchased, coconut oil is classified as SFA-rich, olive and rapeseed as MUFA-rich, and sunflower oil as PUFA-rich oils. However, it should also be noted that rapeseed oil has a much higher level of the omega-3 PUFA linolenic acid (predominantly as triacylglycerols), i.e. approximately 10% (w/w); linolenoylglycerols are, of course, more susceptible to peroxidation than linoleoylglycerols, and give rise to a different pattern of secondary aldehydic LOPs than those derived from the latter source.2.2 Thermal stressing of culinary oil samples
Thermal stressing of coconut, olive, rapeseed and sunflower oils was conducted in the presence of atmospheric oxygen at 180 °C with increasing time periods in three different series of standard heating or cooking practices. For comparative, quantitative 1H NMR evaluations, it was ensured that the same electronic balance (±0.1 g accuracy, Mettler (UK), Model AT261) and electronically-controlled hot-plate (230 V, 50 Hz, 750 W; Stuart heat-stir (UK), Model SB162) were used for the weighing and heating experiments respectively. All glassware was dried under N2 and completely sealed after discharging the amount required for heating.2.3 Continuous heating episodes
The first type of heating, termed “continuous” heating, and initially described in ref. 22 was adapted and slightly modified: 20 g of oil samples were weighed directly into 100 mL beakers of lipid-air surface area 70.69 cm2, and then were heated on an electronically-controlled hot-plate operated at 180 °C for a total duration of 300 min. With the aid of a 230 mm Fischerbrand glass Pasteur pipette, 1.0 mL of thermally-stressed oil was sampled from the hot oil source into glass vials at regular 30 min time intervals for a total period of 300 min (as shown in Fig. 7). Cooling of the sampled oils was conducted rapidly on ice, and they were then processed for rapid 1H NMR measurements. The heating experiment and sampling were repeated in triplicate for each oil evaluated in this manner. Control experiments for the studied culinary oils were their respective unheated samples. The results of all detected, quantified LOPs were presented as mean ± standard deviation (SD) values.2.4 Discontinuous heating and cooling episodes
In the discontinuous heating and cooling series of experiments, the same process outlined in section 2.3 above was followed. These were then heated in a discontinuous fashion for 30 min heating on an electronically-controlled hot-plate plate operated at 180 °C. This was followed by an ambient temperature cooling period of 120 min (Fig. 8). An aliquot of 1.0 mL of thermally-stressed oil was sampled at specified times (shown as in Fig. 4) from the thermally-oxidised oil into glass vials for 1H NMR analysis. Sampled oils were then rapidly cooled on ice. The discontinuous heating and cooling experiment was conducted in triplicate for each culinary oil. Unheated culinary oil samples served as essential controls. All LOP levels reported were expressed as mean ± SD values.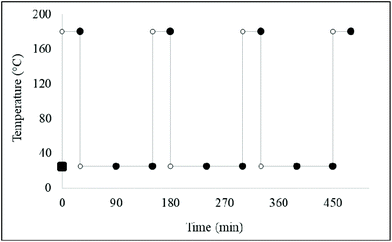 | ||
| Fig. 8 A discontinuous heating and intermittent cooling assay involving a timely sampling method for each thermally-stressed culinary oil investigated. | ||
2.5 1H NMR analysis of LOPs
Changes in the composition of all examined oils were monitored by 1H NMR analysis conducted on a Bruker Avance III 400 MHz (BBI) and 600 MHz (TXI) spectrometers (Kingston University, London) operating at 400.13 and 600.13 MHz frequencies respectively, and a probe temperature of 298 K. For both instruments, the following acquisition parameters were employed: size of fid 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 536; number of scans 256; probe temperature 300 K; spectral width 20.573 ppm; relaxation delay 1.000 s; acquisition time 4.819 s; pulse width 90 °C; and total acquisition time 16 min.
536; number of scans 256; probe temperature 300 K; spectral width 20.573 ppm; relaxation delay 1.000 s; acquisition time 4.819 s; pulse width 90 °C; and total acquisition time 16 min.
An 0.30 mL aliquot of each control (unheated) and thermally-stressed culinary oil was diluted with a 0.60 mL volume of deuterated chloroform (CDCl3) (99.8% purity, Sigma-Aldrich Chemical Co., UK). CDCl3, which served to provide a field frequency lock for the studied oil samples, had a chemical shift (δ) value of 7.283 ppm. An aliquot (0.50 mL) of the resulting lipid-CDCl3 mixture was then pipetted into a 5 mm diameter NMR tube (Norrell HT, GPE Scientific). This was further treated with a 0.10 mL of a solution of 1,3,5-tribromobenzene (Sigma-Aldrich) (TBB) (prepared by dissolving 4.14 mg TBB in 2.0 mL of CDCl3). With a 7.537 ppm δ value, TBB served as an internal quantitative NMR standard in the determination of NMR-detectable peroxidation products in thermally-stressed oil samples. All 1H NMR chemical shifts, regardless of oil nature, were referenced to tetramethylsilane (TMS) (δ = 0.000 ppm) and/or residual chloroform (δ = 7.283 ppm). The 1H NMR spectra obtained in this study were analysed using Topspin 3.5 sp7 (Bruker Biospin), and were found to be fully consistent with literature reports.11,21,23,24
2.6. Analysis of culinary oil LOPs
Lipid oxidation products (LOPs) were identified from 1H NMR spectra acquired in the present study with the aid of literature references.6,23,24 In addition to 1D 1H NMR analysis, carbon-13 (13C) and 13C Distortionless Enhancement by Polarization Transfer (13C DEPT-90) spectra, together with other two-dimensional (2D) NMR spectroscopic analyses, i.e. Heteronuclear Single Quantum Correlation (HSQC) and Correlation Spectroscopy (COSY) were also employed to confirm the molecular identification of LOPs.2.7 Experimental design and statistical analysis
For experiments involving the exposure of CLO products to thermal stressing episodes, the experimental design for univariate analysis of the 1H NMR aldehyde classification concentration datasets involved an analysis-of-covariance (ANCOVA) model, which incorporated 3 prime factors, and a total of 7 sources of variation: (1) that ‘between-oil products’, qualitative fixed effect (Oi); (2) that ‘between-sampling time-points’ quantitative fixed effect ‘nested’ within the culinary oil classifications (Tj); (3) the thermal stressing method employed, i.e. continuous versus discontinuous heating strategies (Mk); (4) the oil classification × sampling time-point first-order interaction effect (OTij); (5) the oil classification × thermal stressing method first-order interaction effect (OMik); (6) the sampling time-point × thermal stressing method interaction effect (TMjk); (7) the ‘between-replicate’ random effect nested within factors, R(ijk)l (1), (2) and (3). The mathematical model for this experimental design is shown eqn (1), in which yijklm represents the (univariate) aldehyde ISB dependent variable values observed, μ its overall population mean value in the absence of any significant, influential sources of variation, and eijklm the unexplained error (residual) contribution.| yijkl = μ + Oi + Tj + Mk + OTij + OMjk + TMjk + R(ijk)l + eijkl | (1) |
ANCOVA was conducted with XLSTAT2016 software (Addinsoft, Paris, France). Post-hoc analysis of significant differences observed between individual CLO products and sampling time-points were performed using Tukey's test.
Further analysis of the univariate aldehydic LOP concentration dataset was performed by comparisons of their least square mean (LSM) values. For example, LSM values for the ‘between-thermal stressing method’ factor were computed by adjusting for the major ‘between oil classifications’ and ‘between-sampling time-point’ sources of variation. The statistical significance of these LSM differences were determined by Tukey's ANCOVA post-hoc test.
2.8 1H NMR analysis of aldehydes in fried potato chip servings
For domestic (‘at-home’) deep-frying episode experiments, batches of hand-cut chips of lengths and widths of 87.00 ± 1.15 and 12.70 ± 0.36 mm (mean ± SEM) respectively were consecutively fried 8 times daily for two days, and then only 3 times on the third day using sunflower oil. The deep-frying facility employed was a domestic model (EasyPro, Tefal) equipped with a variable thermostat and an inert cross-lined steel mesh for the purpose of lowering the chips into the oil without contacting the fryer's inner surface. This deep fryer was filled with 3.00 litres of oil according to the manufacturer's instructions, and 400 ± 10 g of potato chips then deep fried at a temperature of 170 °C for a period of 10.0 min.The % (w/w) total PUFA, MUFA and SFA contents of the sunflower oil employed in these studies was 61.0, 28.0 and 11.0% (w/w) respectively.
Each frying oil used was allowed to cool for a period of exactly 30 min between each repetitive frying episode (a total of 8 per full daily cycle, as noted above). Following each 10 min frying episode, chips were thoroughly shaken in their wire basket for 15 s, and then allowed to drain therein for 30 s to remove excess oil. Chips were then transferred to a steel mesh draining board.
At the final collection time-point on day 3 (corresponding to the 19th frying session), 2 randomly-selected samples of chips were transferred to plastic-stoppered sample tubes and immediately frozen at a temperature of −20 °C until transported to the laboratory where they were then stored at −80 °C for a maximum duration of 18 h prior to 1H NMR analysis. On completion this finalised frying episode, duplicate samples of sunflower oil were also collected for analysis, and these were also stored prior to analysis in the same manner as the potato chip samples, as were duplicate unheated (control) frying oil samples. Two samples of the unfried potatoes were also collected and stored in this manner.
3. Results and discussion
3.1 Culinary oil FA compositions and IV
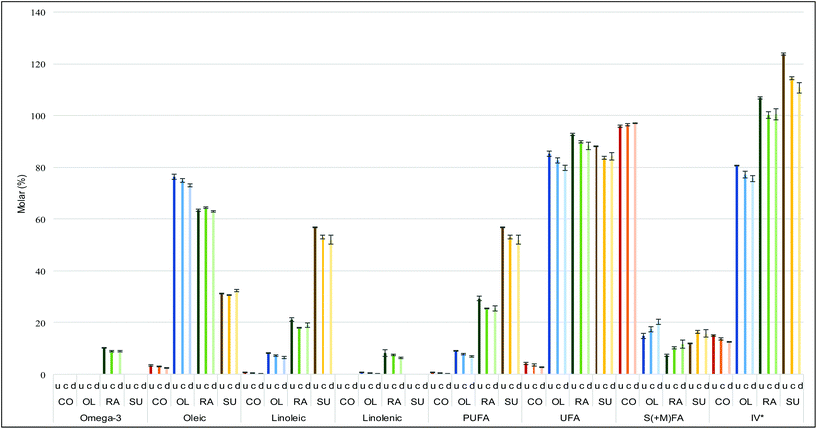
The molar percentages and iodine value of a series of FA acyl groups were deduced from the 1H NMR spectral profiles of the oil samples investigated. These deductions were based on the assumption that the area of the signals generated in each 1H NMR spectrum is proportional to the number of 1H nuclei giving rise to them, and that the proportionality constant is the same in all cases. Acyl groups such as omega-3, oleic (or monounsaturated), linoleic and linolenic (or polyunsaturated), as well as saturated and modified groups were inferred from the 1H NMR spectra acquired (Fig. 1). PUFAs, as well as total unsaturated acyl groups, were calculated from the data generated from the mathematical computations of oleic, linoleic and linolenic acyl groups (ESI Summary S1†). The determination of iodine value (IV) was deduced directly from the olefinic protons observed in the 1H NMR spectra of culinary oils6 (ESI section S1†). All deductions of the acyl group compositions were computed in triplicate, and the results obtained are provided as mean ± SD values (Table 3).6,25Amongst the unsaturated FA-rich oils, the greater the amount of oleic acid, the more resistant the oil to thermo-oxidation,15 although SFA's are considerably more resistant to oxidation than MUFAs. Concentrations of linoleic acyl groups and PUFA groups were in the order sunflower oil > rapeseed oil > olive oil > coconut fat. By implication, sunflower oil was most susceptible to thermodegradation.15,26,27 Amongst the heating systems employed, discontinuous heating episodes, regardless of the culinary oil tested, had the least impact on preoxidation of oil UFAs. Plots comparing individual FA components are provided in the ESI (Table S1 and Fig. S5, S6†).
3.2 Evolution of LOPs
A proposed integrated reaction scheme showing how oxidation products could evolve was adapted from ref. 28 and slightly modified (ESI Fig. S9†). At the pre-heating time-point (0.0 min), only very low levels of LOPs are expected to be present. This was correct for coconut and rapeseed oils. However, unheated olive oil was found to contain relatively low levels of n-alkanals, (E,E)-2,4-alkadienals and (E)-2-alkenals at concentrations of 0.09, 0.02 and 0.09 mM, respectively. Also, n-alkanals (0.19 mM) and (E)-2-alkenals (0.15 mM) were quantifiable in unheated sunflower oil. Prior to the 1H NMR measurements, all culinary oils purchased from the local supermarket were stored in dark for not more than 72 hours.As noted above, (E,E)-2,4-alkadienals and (E)-2-alkenals are both examples of α,β-UAs, and therefore their presence in unheated culinary oil presents a potential health risk to consumers. The presence of LOPs in unheated olive and sunflower oils may be attributed to possible exposure of the oils to variable forms of oxidative stress, through industrial refinement and/or subjection to prolonged storage conditions.5,22,29
The differences in unsaturation degree account for the proportional distribution of aldehydic LOPs between sunflower and olive oil at time 0 min.5 For example, (E,E)-2,4-alkadienals, along with 4,5-epoxy-(E)-2-alkenals derived directly therefrom, arise from the peroxidation of PUFAs, whereas (E)-2-alkenals and higher molecular mass n-alkanals are generated from both MUFAs and PUFAs. Moreover, acrolein, malondialdehyde (MDA) and propanal are generated from omega-3 FAs, almost exclusively from linolenoylglycerols present in vegetable oils, which is particularly notable for rapeseed oil included in the study performed here.
A general summary of observations and rationale for our quantification strategy is provided in the ESI (Summary S2).†
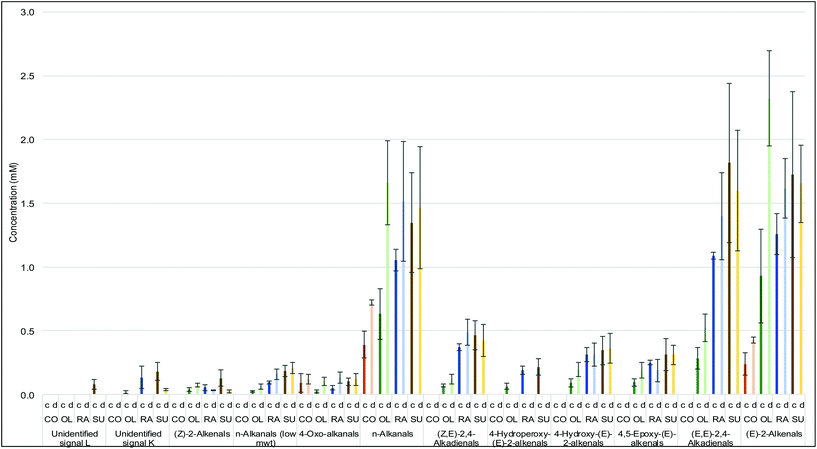
The formation of LOPs and the increases in their concentrations in culinary oils throughout our thermo-oxidation processes can be illustrated in Table 4 and Fig. 6. The intensity of the signals of LOPs generated in the 1H NMR spectra of thermally-stressed culinary oils proportionately correspond to the concentrations of these oxidation products. At a constant temperature of 180 °C, the amounts of LOPs generated increase with time as previously reported.26 Also, the degree of unsaturation had a high level of influence on the evolution of LOPs, as expected.4 By implication, thermally-stressed SFA-rich coconut oil yielded only three types of LOPs, specifically (E)-2-alkenals (signal a), n-alkanals (signal g) and 4-oxo-n-alkanals (signal h). However, the concentrations of these LOPs were all lower in comparison to those of the other, much more MUFA- and/or PUFA-rich oils tested, as expected from its very high SFA content. There were, however, striking similarities between olive oil and rapeseed oil, since both oils are MUFA-rich and therefore share a similar degree of thermo-oxidation resistivity characteristics. Sunflower oil yielded the largest proportion of LOPs over a total 300 min heating period (please refer to ESI Table 2 and Fig. S7, S8†). This included the unsaturated aldehyde doublet k which was absent from the 1H NMR profiles of thermally-stressed coconut oil, as well as those of olive and rapeseed oil. The susceptibility of sunflower oil to thermo-oxidation is certainly attributable to its high content of PUFAs.
| Functional group | ||||
|---|---|---|---|---|
| Signal | Chemical shift (ppm) | Multiplicity | Condensed function | Classification |
| Abbreviation: d, doublet; t, triplet.a First identified by ref. 15. Letter assignments correspond to those in Fig. 5 and 6. | ||||
| a | 9.472–9.505 | d | –CHO | (E)-2-Alkenals |
| b | 9.505–9.535 | d | –CHO | (E,E)-2,4-Alkadienals |
| c | 9.535–9.557 | d | –CHO | 4,5-Epoxy-(E)-alkenals |
| d | 9.557–9.583 | d | –CHO | 4-Hydroxy-(E)-2-alkenals |
| e | 9.570–9.587 | d | –CHO | 4-Hydroperoxy-(E)-2-alkenals |
| f | 9.587–9.612 | d | –CHO | (Z,E)-2,4-Alkadienals |
| g | 9.729–9.755 | t | –CHO | n-Alkanals |
| h | 9.776–9.793 | t | –CHO | 4-Oxo-alkanals |
| i | 9.793–9.809 | t | –CHO | n-Alkanals of low-molecular-mass (propanal and butanal) |
| j | 10.048–10.075 | d | –CHO | (Z)-2-Alkenalsa |
| k | 10.138–10.163 | d | –CHO | Unidentified unsaturated aldehyde |
| l | 10.201–10.225 | d | –CHO | Unidentified unsaturated aldehyde |
To compare variations in LOP composition between our discontinuous and continuous heating protocols, a comparison of the concentrations of all identifiable aldehydic LOPs at a fixed time point (120 min) was undertaken (Fig. 10). Whilst the concentrations of LOPs follow the trend of being proportional to the degree of unsaturation during the continuous heating process, employment of the discontinuous heating strategy exerted a marked impact on their observed concentrations.
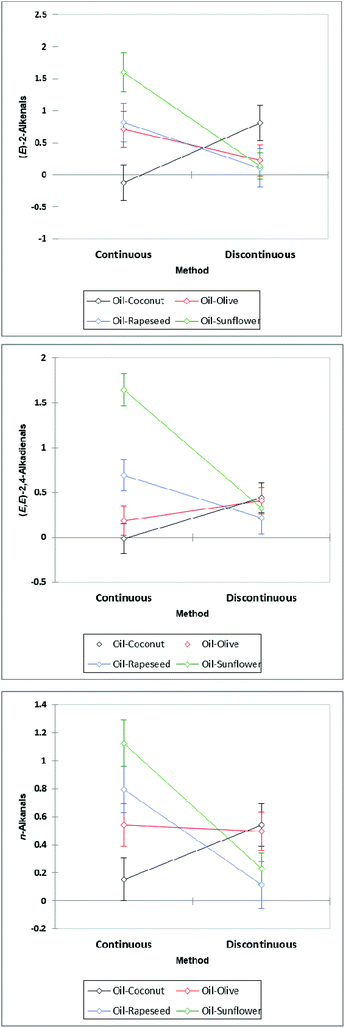
ANCOVA analysis of LSM values revealed that the continuous method of thermal stressing only generated significantly greater levels of aldehyde than the discontinuous approach for two of the aldehydes monitored (p < 5.59 × 10−3 and 0.023 for (E)-2-alkenals low-molecular-mass n-alkanals respectively) (Table 5). However, it was also clear that any ‘between-methods’ differences manifested were markedly influenced by the nature of the oil product tested. Indeed, all thermal stressing method × oil nature interaction effects in this model were also very highly significant (p < 10−6 for all aldehydes tested), and from Fig. 11, is clear that although sunflower and rapeseed oils generated much lower levels of aldehydic LOPs with the discontinuous heating approach, this method gave rise to higher levels of these toxins in coconut oil; in general, there appeared to be no major differences between these two methods for olive oil.
| U2 | U1 | (Z)-2-Alkenals | n-Alkanals (low m.wt) | 4-Oxo-n-alkanals | n-Alkanals | (Z,E)-2,4-Alkadienals | 4-Hydroperoxy-(E)-2-alkenals | 4-Hydroxy-(E)-2-alkenals | 4,5-Epoxy-(E)-alkenals | (E,E)-2,4-Alkadienals | (E)-2-Alkenals | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbreviations: Unidentified signal 1 (U1), Unidentified signal 2 (U2). | ||||||||||||
| R 2 | 0.69 | 0.83 | 0.79 | 0.87 | 0.83 | 0.85 | 0.88 | 0.82 | 0.85 | 0.83 | 0.85 | 0.83 |
| Overall model | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 |
| Time-point | 2.68 × 10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 |
| Method | 0.15 | 0.39 | 0.15 | 0.023 | 0.11 | 0.10 | 0.60 | 0.077 | 0.12 | 0.13 | 0.41 | 5.59 × 10−3 |
| Oil | 5.77 × 10−5 | <10−6 | 7.49 × 10−5 | <10−6 | 2.81 × 10−2 | 2.33 × 10−5 | <10−6 | 1.62 × 10−6 | <10−6 | 9.71 × 10−6 | <10−6 | 1.05 × 10−3 |
| Time-point × method | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 |
| Time-point × oil | <10−6 | <10−6 | <10−6 | <10−6 | 1.19 × 10−4 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 |
| Method × oil | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 | <10−6 |
In the case of both olive and rapeseed oils, the discontinuous heating process produces higher concentrations of low-molecular-mass n-alkanals, 4-oxo-n-alkanals, n-alkanals and (E,E)-2,4-alkadienals at the same time point when compared to those observed during the continuous heating protocol. This suggests a temperature independence of these LOPs being generated (see Fig. S7 and S8 in ESI†). In practical culinary terms, the heating and cooling of these two oils may result in higher concentrations of these LOPs than by their continuous heating alone. This was to be expected, since during your oil ‘rest’ periods, the temperature is still quite high, and it takes some time for oil temperature to return to an ambient value. Since MUFA oxidation is a lot slower than that of PUFAs, it also provides more time for this to occur. Interestingly, there was no significant difference between LOPs formed in sunflower oil between discontinuous and continuous heating processes. PUFA-rich sunflower oil therefore may possess a greater temperature-independent resistance to oxidation in view of the presence of antioxidants such as alpha-tocopherol (mM levels), which are much more effective against peroxidation at lower temperatures. Coconut oil yielded only very low concentrations of LOPs by NMR analysis when heated according to either process.
Fig. 12(a) shows a typical aldehyde-CHO function regions of the 1H NMR profiles of a C2HCl3 extract of potato chips deep-fried in sunflower when subjected to a repetitive cycles of 19 consecutive 10 min deep-frying episodes within a domestic deep fryer facility at 170 °C according to section 2.8. Corresponding partial spectra of the sunflower oil utilised for this purpose is displayed in Fig. 4(b). Aldehydes detectable in the potato chip samples include (E)-2-alkenals, (E,E)-alka-2,4-dienals, 4,5-epoxy-trans-2-alkenals, a combination of 4-hydroxy-/4-hydroperoxy-trans-2-alkenals, (Z,E)-alka-2,4-dienals and n-alkanals, along with two unassigned aldehydic proton resonances in the profile shown in (a). Moreover, Fig. 12(c) shows the corresponding 1H NMR spectral region for a sample of fried potato chips obtained from a local Chinese take-out vendor.
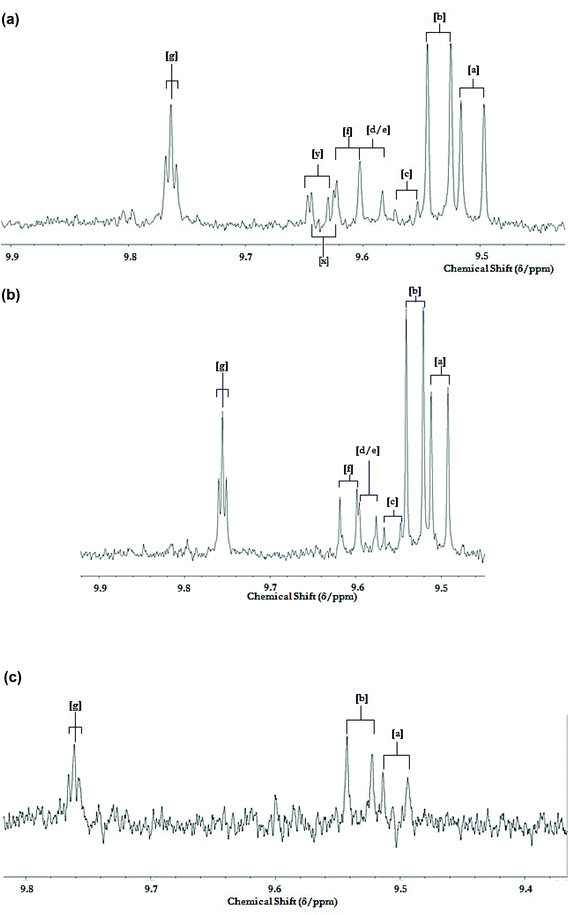 | ||
| Fig. 12 1H NMR analysis of potato chips deep-fried according to repetitive domestic deep repetitive frying sessions. (a) Partial 9.40–9.90 ppm regions of the 1H NMR profile of a C2HCl3 extract of potato chip samples deep-fried in sunflower oil exposed to 2× repetitive cycles of 8 sequential 10 min length deep-frying episodes in a commercially-available domestic deep fryer unit at 170 °C for two days, followed by 3 further 10 min frying episodes on day 3 (19 in total). Potato chip samples were fried, collected and extracted by the method described in section 2.8. (b) Corresponding partial spectra of the sunflower oil used for these sequential deep-frying sessions; samples were collected for 1H NMR analysis immediately on completion of the above 19th 10 min frying session. (c) Corresponding partial spectra of a C2HCl3 extract of a potato chip sample purchased from a local take-out restaurant. Abbreviations: aldehyde-CHO function resonance assignments a, b, c, d, e, f and g correspond to those in Fig. 5. Resonances labelled x and y tentatively arise from two unassigned classes of α,β-unsaturated aldehydes. | ||
4. Conclusions
This manuscript compares, for the first time, the effects of continuous and discontinuous heating strategies on the LOP/FA composition of saturated- and poly/monounsaturated-rich oils using high-resolution (600 MHz) 1H NMR analysis in order to characterise and quantitate the different agents in situ, in a non-destructive fashion. Analysis of differences in acylglycerols indicated that the greatest difference observed between continuous and discontinuous heating processes was for sunflower and rapeseed oils. The susceptibility of the selected oils to thermal oxidation has been evaluated via the characterisation of LOPs from a variety of PUFA, MUFA and SFA sources, with coconut oil exhibiting the most heat resistant activities in this study, with sunflower oil being the most susceptible, as expected. The impact of heating protocol on the identities and quantities of LOPs evolving throughout a 2.0 h period implies a degree of innate resistance to discontinuous heat-induced autocatalytic oxidation cycles by sunflower oil, when compared to the effects observed with rapeseed and olive oil media. This observation is particularly noteworthy in view of the expectation that this susceptibility would be linked to the degree of unsaturation of the cooking oils investigated.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors are grateful to Doctoral Training Alliance and Kingston University for their support for this project.References
- A. Catalá, Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions, Chem. Phys. Lipids, 2009, 157, 1–11 CrossRef PubMed.
- J. Alberdi-Cedeño, M. L. Ibargoitia, G. Cristillo, P. Sopelana and M. D. Guillén, A new methodology capable of characterizing most volatile and less volatile minor edible oils components in a single chromatographic run without solvents or reagents. Detection of new components, Food Chem., 2017, 221, 1135–1144 CrossRef PubMed.
- M. Grootveld, C. J. L. Silwood, P. Addis and A. Claxson, Health effects of oxidized heated oils, Food Res. Int., 2001, 13, 41–55 CrossRef.
- R. P. Venkata and R. Subramanyam, Evaluation of the deleterious health effects of consumption of repeatedly heated vegetable oil, Toxicol. Rep., 2016, 3, 636–643 CrossRef PubMed.
- M. Grootveld, V. R. Rodado and C. J. L. Silwood, Detection, monitoring, and deleterious health effects of lipid oxidation products generated in culinary oils during thermal stressing episodes, InForm, 2014, 25, 614–624 Search PubMed.
- A. Martínez-Yusta, E. Goicoechea and M. D. Guillén, A review of thermo-oxidative degradation of food lipids studied by 1H NMR spectroscopy: influence of degradative conditions and food lipid nature, Compr. Rev. Food Sci. Food Saf., 2014, 13, 838–859 CrossRef.
- M. D. Guillén and A. Ruiz, Rapid simultaneous determination by proton NMR of unsaturation and composition of acyl groups in vegetable oils, Eur. J. Lipid Sci. Technol., 2003, 105, 688–696 CrossRef.
- M. D. Guillén and P. S. Uriarte, Simultaneous control of the evolution of the percentage in weight of polar compounds, iodine value, acyl groups proportions and aldehydes concentrations in sunflower oil submitted to frying temperature in an industrial fryer, Food Control, 2012a, 24, 50–56 Search PubMed.
- M. D. Guillén and P. S. Uriarte, Contribution to further understanding of the evolution of sunflower oil submitted to frying temperature in a domestic fryer: study by 1H Nuclear Magnetic Resonance, J. Agric. Food Chem., 2009, 57, 7790–7799 CrossRef PubMed.
- E. Goicoechea and M. D. Guillén, Analysis of hydroperoxides, aldehydes and epoxides by 1H Nuclear Magnetic Resonance in sunflower oil oxidized at 70 and 100 °C, J. Agric. Food Chem., 2010, 58, 6234–6245 CrossRef CAS PubMed.
- M. D. Guillén and E. Goicoechea, Oxidation of corn oil at room temperature: primary and secondary oxidation products and determination of their concentration in the oil liquid matrix from 1H Nuclear Magnetic Resonance data, Food Chem., 2009, 116, 183–192 CrossRef.
- M. D. Guillén and P. S. Uriarte, Study by 1H NMR spectroscopy of the evolution of extra virgin olive oil composition submitted to frying temperature in an industrial fryer for a prolonged period of time, Food Chem., 2012b, 134, 162–172 Search PubMed.
- B. M. Markaverich, J. R. Crowley, M. A. Alejandro, K. Shoulars, N. Casajuna, S. Mani, A. Reyna and J. Sharp, Leukotoxins diols from ground corncob bedding disrupt estrus cyclicity in rats and stimulate MCF-7 breast cancer cell proliferation, Environ. Health Perspect., 2005, 113, 1698–1704 CrossRef CAS PubMed.
- D. A. Thompson and B. D. Hammock, Dihydroxyoctadecamonoenoate esters inhibit the neutrophil respiratory burst, J. Biosci., 2007, 32, 279–291 CrossRef CAS PubMed; B. Chen, A. Han, D. J. McClements and E. A. Decker, Physical structures in soybean oil and their impact on lipid oxidation, J. Agric. Food Chem., 1994, 58, 11993–11999 CrossRef PubMed.
- S. Moumtaz, B. C. Percival, D. Parmar, K. L. Grootveld, P. Jansson and M. Grootveld, Toxic aldehyde generation in and food uptake from culinary oils during frying practices: peroxidative resistance of a monounsaturate-rich algae oil, Sci. Rep., 2019, 9, 1–21 CrossRef CAS PubMed.
- M. J. Picklo, A. Azenkeng and M. R. Hoffmann, Trans-4-oxo-2-nonenal potently alters mitochondrial function, Free Radicals Biol. Med., 2011, 50, 400–407 CrossRef CAS PubMed.
- S. P. Hussain, L. S. Hofseth and C. C. Harris, Radical causes of cancer, Nat. Rev. Cancer, 2003, 3, 276–285 CrossRef CAS PubMed.
- R. Guillén-Sans and M. Guzman-Chozas, The thiobarbituric acid (TBA) reaction in foods: a review, Crit. Rev. Food Sci., 1998, 38, 315–330 CrossRef PubMed.
- M. Laguerre, J. Lecomte and P. Villeneuve, Evaluation of the ability of antioxidants to counteract lipid oxidation: existing methods, new trends and challenges, Prog. Lipid Res., 2007, 46, 244–282 CrossRef CAS PubMed.
- A. Le Gresley and J. M. Peron, A semi-automatic approach to the characterisation of dark chocolate by Nuclear Magnetic Resonance and multivariate analysis, Food Chem., 2019, 275, 385–389 CrossRef CAS PubMed.
- E. Falcha, H. W. Anthonsenc, D. E. Axelson and M. Aursanda, Correlation between 1H NMR and traditional methods for determining lipid oxidation of ethyl docosahexaenoate, J. Am. Oil Chem. Soc., 2004, 81, 1105–1110 CrossRef.
- A. W. D. Claxson, G. E. Hawkes, D. P. Richardson, D. P. Naughton, R. M. Haywood, C. L. Chander, M. Atherton, E. J. Lynch and M. Grootveld, Generation of lipid peroxidation products in culinary oils and fats during episodes of thermal stressing: a high field 1H NMR study, FEBS Lett., 1994, 355, 81–90 CrossRef CAS PubMed.
- A. Martínez-Yusta and M. D. Guillén, Monitoring compositional changes in sunflower oil-derived deep-frying media by 1H Nuclear Magnetic Resonance, Eur. J. Lipid Sci. Technol., 2016, 118, 984–996 CrossRef.
- B. Nieva-Echevarría, E. Goicoechea and M. D. Guillén, Behaviour of non-oxidized and oxidized flaxseed oils, as models of omega-3 rich lipids, during in vitro digestion. Occurrence of epoxidation reactions, Food Res. Int., 2017, 97, 104–115 CrossRef PubMed.
- B. Nieva-Echevarría, E. Goicoechea, M. J. Manzanos and M. D. Guillén, The influence of frying technique, cooking oil and fish species on the changes occurring in fish lipids and oil during shallow-frying, studied by 1H NMR, Food Res. Int., 2016, 84, 150–159 CrossRef.
- M. D. Guillén and E. Goicoechea, Detection of primary and secondary oxidation products by Fourier Transform Infrared Spectroscopy (FTIR) and 1H Nuclear Magnetic Resonance (NMR) in sunflower oil during storage, J. Agric. Food Chem., 2007, 55, 10729–10736 CrossRef PubMed.
- M. Grootveld, B. C. Percival and K. L. Grootveld, Chronic non-communicable disease risks presented by lipid oxidation products in fried foods, Hepatobil. Surg. Nutr., 2018, 7, 305–312 CrossRef PubMed.
- K. M. Schaich, Thinking outside the classical chain reaction box of lipid oxidation, Lipid Technol., 2012, 24, 55–58 CrossRef CAS.
- R. M. Haywood, A. W. D. Claxson, G. E. Hawkes, D. P. Richardson, D. P. Naughton, G. Coumbarides, J. Hawkes, E. J. Lynch and M. C. Grootveld, Detection of aldehydes and their conjugated hydroperoxydiene precursors in thermally-stressed culinary oils and fats: investigations using high resolution proton NMR spectroscopy, Free Radic. Res., 1995, 22, 441–482 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9fo02065a |
| This journal is © The Royal Society of Chemistry 2019 |