Open Access Article
Open Access ArticleDose–response relationship between cocoa flavanols and human endothelial function: a systematic review and meta-analysis of randomized trials†
Ye
Sun
a,
Diane
Zimmermann
 b,
Carlos Antonio
De Castro
a and
Lucas
Actis-Goretta
b,
Carlos Antonio
De Castro
a and
Lucas
Actis-Goretta
 *a
*a
aNestlé Research Centre Singapore, Singapore. E-mail: lucas.actisgoretta@rdsg.nestle.com; Tel: +65 6635 2242
bNestlé Research Centre Lausanne, Switzerland
First published on 16th September 2019
Abstract
Background: Several intervention studies have investigated the relationship between cocoa flavanols and endothelial function. However, the shape of the association and the type of compounds responsible for the effects are largely unknown. Objective: To examine the dose–response association between the consumption of cocoa flavanols and endothelial function, measured by flow-mediated dilation (FMD). Design: Two investigators searched Scopus® for the relevant human intervention studies, which were pooled and meta-analysed. Heterogeneity in the findings was explored with various subgroup analyses. Results: Fifteen published articles with 18 intervention arms met the inclusion criteria. Participants in these intervention groups received 80 to 1248 mg (mean: 704 mg) more flavanols than control groups. A significant improvement of FMD by 1.17% (95% CI: 0.76% to 1.57%) was calculated, with strong evidence of a non-linear association (inverted U-shape) between cocoa flavanols and FMD. Conclusions: This meta-analysis provides evidence that cocoa flavanols could significantly improve endothelial function, with an optimal effect observed with 710 mg total flavanols, 95 mg (−)-epicatechin or 25 mg (+)-catechin. However, there was substantial variation in the results that could not be explained by the characteristics that we explored, and there were significant risk-of-bias concerns with a large majority of the studies.
Introduction
Observational studies suggest that consumption of flavanol-rich products, such as dark chocolate, is associated with a reduced risk of developing cardiovascular disease.1,2 Additionally, a number of human randomized clinical trials (RCTs) have convincingly shown a vascular benefit from consumption of flavanol-rich products in different population groups. Meta-analyses of such clinical trials have demonstrated that consumption of flavanol-rich products improved endothelial dysfunction as assessed by flow-mediated dilation (FMD).3,4Whilst, from a nutritional perspective, it is often more meaningful to investigate the impact of foods rather than nutrients,5 sometimes nutrient analysis is necessary to gain insight into the main bioactive constituents, in order to be able to recommend foods comparably rich in the most prominent bioactive(s).6 Nutrient analysis of cocoa flavanols shows that they comprise several types of compounds such as (−)-epicatechin, (+)-catechin and their oligomers, called procyanidins (degree of polymerization from n = 2 to n = 10).7 Although few studies have documented the generation of absorbable compounds by microbiological biotransformation of procyanidins in the large intestine,8 most evidence suggests that essentially monomers and, to a very low extent dimers, are absorbed in the small intestine;7 among them, (−)-epicatechin would be primarily responsible for their biological activity.
Acute intervention trials have demonstrated improvement in FMD after consumption of 1–2 mg per kg body weight (up to 200 mg) of purified (−)-epicatechin9,10 while a 30-day intervention trial with 100 mg of purified (−)-epicatechin failed to show any improvement in the same parameter.11 In a previous meta-analysis, Hooper et al. 2012![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 observed improvement in FMD by chocolate/cocoa consumption both acutely and chronically, with no apparent dose-effect relationship.
3 observed improvement in FMD by chocolate/cocoa consumption both acutely and chronically, with no apparent dose-effect relationship.
A number of articles investigating this area have been published since then and, with this background, we aimed to perform an updated systematic review and meta-analysis to summarize the current scientific evidence, and evaluate the main bioactive(s) and their optimal doses from cocoa/chocolate to positively influence endothelial function assessed by FMD.
Subjects and methods
Data sources and search strategy
A literature search was performed independently by two investigators by means of the Scopus® database (Elsevier BV, Amsterdam, The Netherlands), to identify RCTs published up to January 2019 that investigated the effects of daily consumption of flavanol-rich cocoa products on endothelial function. Key words used in the search criteria were:(“Arterial function” OR “vascular function” OR “vascular dysfunction” OR “flow mediated dilat*” OR “flow mediated vasodilat*” OR “endothel* dependent dilat*” OR “endothel* dependent vasodilat*” OR flow-mediated OR “flow mediated” OR brachial reactivity OR FMD) AND (cocoa* OR chocolate OR cacao) AND (random* control* trial OR control* clinical trial OR trial OR “double blind*” OR “single blind*”)
In addition, reference lists of published trials and reviews were checked.
Literature selection
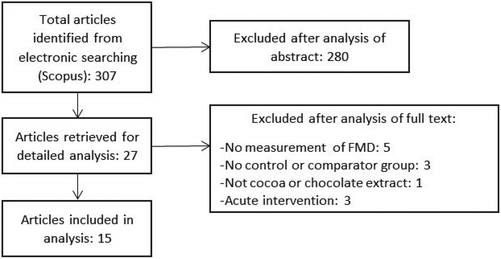
RCTs were included in the meta-analysis if they met the following inclusion criteria: the primary or secondary outcome of the study was FMD; the intervention lasted for at least 1 week; the intervention was a flavanol-containing cocoa/chocolate food; the last measurement of FMD was performed in a fasted condition after the intervention period. A flow diagram for study selection is presented in Fig. 1.Data extraction
Following the literature search, the investigators reviewed the study titles and abstracts followed by the full-text articles for eligibility. Discrepancies were resolved by discussion and agreement. Data were extracted following the inclusion criteria; studies were also excluded if there was no FMD measurement, no control or comparator group, did not include cocoa or chocolate extract, or when acute intervention was performed. Data on means and standard deviations (SD) at the end of the intervention for both treatment and control groups were extracted. If FMD data were not available in the text, they were estimated from the plots. If SDs were not reported, they were calculated or estimated from standard errors (SEs), confidence intervals (CIs), p-values for difference in means, or pooled correlation coefficients between baseline and final measurements from trials reporting sufficient information. Multiple comparison arms sharing the same control group were combined to create a single pair-wise comparison to avoid double counting and correlated comparisons. If more than one flavanol dose levels were tested, only the results from the highest dose arm were included in the main meta-analysis, but all arms with different doses were included in the subgroup meta-analysis by dose of flavanols and in the dose–response analysis. If more than one treatment time was described in the intervention (i.e. 7 and 14 days), only the longest intervention time was considered for the main meta-analysis, but all time-points were used in the subgroup meta-analysis grouped by duration of treatment.Risk of bias assessment
To assess the risk of bias, two investigators independently performed a risk-of-bias assessment using the Cochrane Collaboration's tool.12 The criteria to assess risk of bias were random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data or selective reporting. Overall risk of bias was defined as low when 4–6 of these criteria scored low risk and 0–2 criteria scored unclear risk. Moderate risk of bias was defined as unclear risk in 3–4 of the criteria, low risk in 2–3 criteria, and moderate risk in 0–1 criteria. Trials with high risk assessment in any of the criteria were considered as high risk.Statistical analysis
All statistical analyses for the meta-analysis were performed using the open-source statistical software R ver. 3.2.3 and Stata version 15 (StataCorp LLC, Texas, USA). The meta-analysis was performed using the function metacont() within the package meta which produces both fixed- and random-effects estimates with continuous outcome data. Inverse variance weighting was used to pool the different studies/publications.13 Results from the random-effects model were presented, considering the large between-study heterogeneity in most cases. Sensitivity analyses were conducted with a fixed-effects model to compare different models. The graphical representation and smoothing curve were done with package ggplot2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14,15 with the function geom_smooth().
14,15 with the function geom_smooth().
Locally weighted scatterplot smoothing (loess) function was used since the number of observations was below 1000. Weights were applied using the ones from the random-effects model of the meta-analysis (due to relatively high heterogeneity). Heterogeneity in the study results was quantified by the I2 statistic.14 Potential sources of heterogeneity were investigated by stratified meta-analyses according to various study characteristics defined a priori: study design, subject characteristics, type of intervention, duration of intervention, risk of bias, and funding body.
Meta-regression was used to assess the statistical significance of the differences across strata. Residual heterogeneity was also assessed using I2 after each of the above-mentioned characteristics were accounted for in meta-regression. Sensitivity analyses were performed to assess the robustness of the meta-analysis by removing the studies one at a time. Publication bias was assessed by visual inspection of the funnel plot,16 by Begg's test17 and Egger's test.18
Results
Study characteristics
The literature search identified 307 articles of which 15 human clinical trials met the inclusion criteria (Fig. 1). The trials included 730 participants in 18 different intervention arms.19–33 The characteristics of these clinical trials are displayed in Table 1. Nine trials had cocoa drink intervention, five investigated chocolate as intervention and the remaining one had a combination of cocoa drink plus chocolate intervention. Trial intervention duration ranged from 7 to 84 days (median: 28 days). The level of flavanol intake in these studies ranged from 80 to 1248 mg (mean: 704 mg).| First author | Year | nC/nT | Age (years) | % Men | Healthy status | Control | Treatment | Duration (days) | ΔTotal flavanols (mg) |
|---|---|---|---|---|---|---|---|---|---|
| Abbreviations: nC: number of subjects in control arm, nT: number of subjects in treatment arm, ΔTotal flavanols: the difference in amount of total flavanols between the treatment and the control, CAD: coronary artery disease, CHF: congestive heart failure, IGT: impaired glucose tolerance. | |||||||||
| Balzer19 | 2008 | 20/21 | 64 ± 8.3 | 29 | Medicated diabetic | Cocoa drink | Cocoa drink | 8, 30 | 888 |
| Davison (A)20 | 2008 | 13/13 | 45 ± 3.5 | 38 | Unhealthy | Cocoa drink and exercise | Cocoa drink and exercise | 84 | 866 |
| Davison (B)20 | 2008 | 11/12 | 45 ± 4.4 | 30 | Unhealthy | Cocoa drink | Cocoa drink | 84 | 866 |
| Esser21 | 2014 | 41/41 | 63 ± 5 | 100 | Overweight | Chocolate | Dark chocolate | 28 | 819 |
| Farouque22 | 2006 | 20/20 | 61 ± 8 | 75 | CAD | Cocoa drink + Chocolate | Cocoa drink + Chocolate | 21, 42 | 424.4 |
| Flammer23 | 2012 | 10/10 | 60 ± 10.1 | 85 | CHF | Chocolate | Chocolate | 14, 28 | 1248 |
| Grassi (A)24 | 2005 | 15/15 | 44 ± 7.8 | 50 | Hypertensive | White chocolate | Chocolate | 15 | 550 |
| Grassi (B)24 | 2005 | 15/15 | 34 ± 7.6 | 47 | Normotensive | White chocolate | Chocolate | 15 | 550 |
| Grassi25 | 2008 | 19/19 | 45 ± 8 | 58 | Hypertensive IGT | White chocolate | Chocolate | 15 | 1008 |
| Grassi26 | 2015 | 20/20 | 54 ± 8.9 | 55 | Healthy | Cocoa drink | Cocoa drink | 7 | 80, 200, 500, 800 |
| Heiss27 | 2010 | 16/16 | 64 ± 12 | 81 | CAD | Cocoa drink | Cocoa drink | 30 | 750 |
| Heiss (A)28 | 2015 | 11/11 | 26 ± 3.2 | 100 | Healthy | Cocoa drink | Cocoa drink | 14 | 900 |
| Heiss (B)28 | 2015 | 10/10 | 60 ± 6 | 100 | Healthy | Cocoa drink | Cocoa drink | 14 | 900 |
| Mogollon29 | 2013 | 20/22 | 29 ± 3.2 | 0 | Pregnant healthy | Chocolate | Chocolate | 42, 84 | 340 |
| Njike30 | 2011 | 39/39 | 52 ± 10.7 | 15 | Overweight | Cocoa drink | Cocoa drink (with/without sugar) | 42 | 796 |
| Sansone31 | 2015 | 50/50 | 45 ± 8 | 50 | Healthy | Cocoa drink | Cocoa drink | 28 | 900 |
| Wang-Polagruto32 | 2006 | 8/9 | 58 ± 2.2 | 0 | Hyper-cholesterolemia | Cocoa drink | Cocoa drink | 42 | 403 |
| Rassaf33 | 2016 | 25/24 | 65 ± 14 | 73 | Haemodialysis | Cocoa drink | Cocoa drink | 30 | 900 |
Endothelial dysfunction assessed by FMD after ingestion of cocoa drink, chocolate, or combination
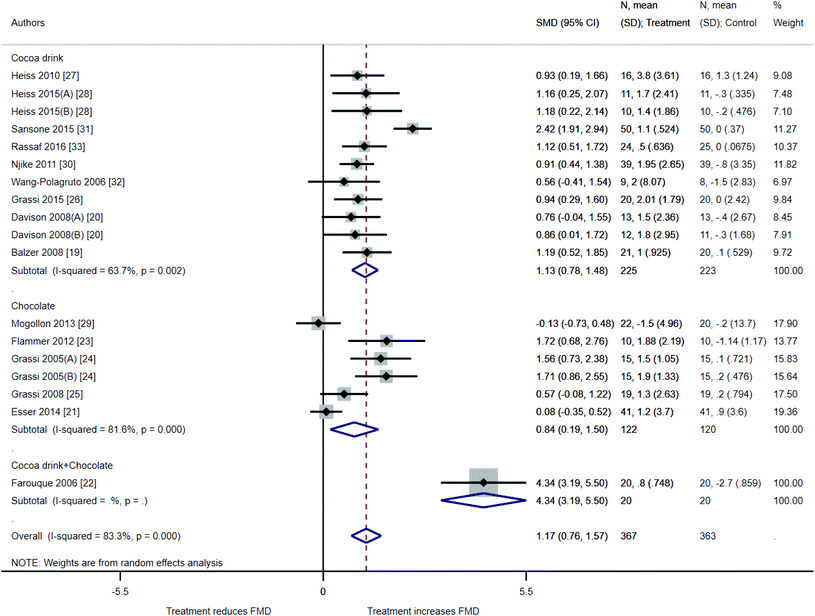
The results of our meta-analysis suggested an improvement in FMD values after ingestion of cocoa drink, chocolate, or a combination of both (1.17%, 95% CI: 0.76%, 1.57%, 18 treatments, 367 participants) (Fig. 2). The high I2 value (I2 = 83.3%) indicates that most of the variability across studies is due to heterogeneity rather than chance. Studies with cocoa drink intervention presented an average FMD improvement of 1.13% (CI of 0.78% and 1.48%) while studies with chocolate showed an FMD improvement of 0.84% (CI of 0.19% and 1.50%). Studies with cocoa drink intervention had a lower heterogeneity than those with chocolate intervention (I2 = 63.7% vs. I2 = 81.6%). The only study with a combination of cocoa drink plus chocolate22 showed a higher FMD improvement (4.34%, 95% CI: 3.19%, 5.50%).Subgroup analyses and meta-regression
Subgroup analyses categorized by interventional treatment, trial design, trial intervention duration, doses per day (from 1 to 3), BMI, age of population treated, potential risk of bias, and source of study funding included in the meta-analysis are depicted in Table 2. The study by Farouque et al.22 combining cocoa drink plus chocolate intervention significantly differed from those studies with cocoa drink or chocolate only (p < 0.001). Studies with low risk of bias demonstrated a smaller but statistically significant effect on FMD (0.78%, CI: 0.38%–1.19%) than studies with moderate risk of bias (p = 0.07). No significant differences were observed between other subgroup categories. Remaining heterogeneity after subgroup meta-analyses showed that only trial intervention categorization accounted for a substantial amount of study heterogeneity (I2 was reduced from 83.3% in the overall studies to 72.6%). Specifically, between-study heterogeneity was reduced to 0% for the following subgroups: studies with treatment duration of 7–15 days (6 studies); studies with mean subject BMI >30 kg m−2 (2 studies); and, studies with mean subject age ranging from 45–60 years (7 studies). The remaining subgroups studied did not demonstrate a major improvement in heterogeneity. No conclusions could be inferred from source of funding since only a small number of studies are grouped in each subgroup.| Group | Arms (n) | Sample size treatment arm (n) | Net change (95% CI) | I 2 (%) | P difference | I 2 remaining after meta-regression (%) | |
|---|---|---|---|---|---|---|---|
| a Balzer 2008A,19 Flammer 2012B23 (<15 days), Balzer 2008B,19 Flammer 2012A,23 Farouque 200622 (<15–30 days), and Farouque 2006A22 (>30 days) are counted in different duration categories. b Heiss 2015B28 (45–60 years) and Heiss 2015A28 (<45 years) are counted in different age categories. c ARC (American Research Cocoa). | |||||||
| All | Total | 18 | 367 | 1.17 [0.76; 1.57] | 83.3 | ||
| Intervention | Cocoa drink | 11 | 225 | 1.13 [0.78; 1.48] | 63.7 | Ref | 72.6 |
| Chocolate | 6 | 122 | 0.84 [0.19; 1.50] | 81.6 | 0.35 | ||
| Both | 1 | 20 | 4.35 [3.19; 5.50] | NA | 0.001 | ||
| Study design | Crossover | 7 | 165 | 0.89 [0.47; 1.31] | 68.6 | Ref | 82.5 |
| Parallel | 11 | 202 | 1.35 [0.72; 1.97] | 86.2 | 0.38 | ||
| Duration | 7–15 days | 8a | 121 | 1.07 [0.80; 1.35] | 0 | Ref | 82.3 |
| >15–30 days | 7a | 182 | 1.32 [0.62; 2.03] | 88.5 | 0.68 | ||
| >30 days | 7a | 137 | 0.99 [0.21; 1.77] | 87.8 | 0.74 | ||
| Number of doses per day | 1 | 4 | 70 | 2.07 [0.84; 3.30] | 88.1 | Ref | 82.4 |
| 2 | 12 | 254 | 1.01 [0.59; 1.44] | 78.6 | 0.07 | ||
| 3 | 2 | 43 | 0.52 [−0.77; 1.81] | 87.8 | 0.06 | ||
| BMI (kg m−2) | 25–30 | 15 | 321 | 1.22 [0.74; 1.7] | 86.1 | Ref | 84.3 |
| >30 | 3 | 46 | 0.97 [0.53; 1.41] | 0 | 0.66 | ||
| Age (years old) | <45 | 6b | 132 | 1.21 [0.35; 2.07] | 89.0 | Ref | 84.5 |
| 45–60 | 8b | 137 | 0.98 [0.72; 1.23] | 0 | 0.70 | ||
| >60 | 4 | 98 | 1.55 [0.18; 2.92] | 93.8 | 0.70 | ||
| Risk of bias assessment | Low risk | 8 | 193 | 0.78 [0.38; 1.19] | 71.1 | Ref | 78.5 |
| Moderate risk | 6 | 116 | 1.76 [0.82; 2.70] | 87.1 | 0.07 | ||
| High risk | 4 | 58 | 1.09 [0.47; 1.70] | 56.3 | 0.61 | ||
| Funding body | Mars | 10 | 186 | 1.42 [0.84; 2.00] | 82.0 | Ref | 80.2 |
| Barry Callebaut | 2 | 42 | 0.40 [−0.65; 1.45] | 82.0 | 0.20 | ||
| Ritter/Kraft | 2 | 30 | 1.63 [1.04; 2.22] | 0.0 | 0.79 | ||
| Hershey | 1 | 39 | 0.91 [0.44; 1.38] | NA | 0.61 | ||
| Cuorenero Sugar Co. | 1 | 19 | 0.57 [−0.08; 1.22] | NA | 0.42 | ||
| ARCc | 1 | 41 | 0.08 [−0.35; 0.52] | NA | 0.20 | ||
| Nestlé | 1 | 10 | 1.72 [0.68; 2.76] | NA | 0.79 | ||
Since cocoa-derived products do not contain similar amounts and profiles in flavanols, subgroup analyses were performed with different dose categories for total flavanols, (−)-epicatechin and (+)-catechin respectively (Table 3). According to the categories described, maximum effect on FMD was observed in studies evaluating over 900 mg of total flavanols per day, 50–<150 mg (−)-epicatechin per day or 20–40 mg (+)-catechin per day. Treatments with 50–<150 mg (−)-epicatechin per day showed a statistically significant difference compared with treatments containing <50 mg day−1 (p = 0.05).
| Group | Arms (n) | Sample size treatment arm (n) | Net change (95% CI) | I 2 (%) | P difference | I 2 remaining after meta-regression (%) | |
|---|---|---|---|---|---|---|---|
| a Grassi 2015D (800–<900 mg day−1), Grassi 2015A, Grassi 2015B and Grassi 2015C (500–<800 mg day−1) are counted in different dose categories. b Grassi 2015D (>40 mg day−1), Grassi 2015C (20–40 mg day−1), Grassi 2015A and Grassi 2015B (0–<20 mg day−1) are counted in different dose categories. c Grassi 2015D (>150 mg day−1), Grassi 2015C (50–150 mg day−1), Grassi 2015A and Grassi 2015B (0–<50 mg day−1) are counted in different dose categories. | |||||||
| All | Total | 18 | 367 | 1.17 [0.76; 1.57] | 83.3 | ||
| Total flavanols | 0–<500 mg day−1 | 5 | 91 | 1.14 [0.01; 2.28] | 91.2 | Ref | 79.4 |
| 500–<800 mg day−1 | 5a | 105 | 1.10 [0.80; 1.41] | 6.6 | 0.83 | ||
| 800–<900 mg day−1 | 5a | 107 | 0.72 [0.25; 1.19] | 60.2 | 0.59 | ||
| >900 mg day−1 | 6 | 124 | 1.37 [0.73; 2.01] | 78.1 | 0.59 | ||
| (−)-Epicatechin | 0–<50 mg day−1 | 4b | 101 | 0.54 [0.08; 1.00] | 59.5 | Ref | 75.1 |
| 50–<150 mg day−1 | 12b | 219 | 1.48 [0.97; 1.98] | 79.9 | 0.05 | ||
| >150 mg day−1 | 5b | 107 | 0.72 [0.25; 1.19] | 60.2 | 0.68 | ||
| (+)-Catechin | 0–<20 mg day−1 | 8c | 173 | 1.00 [0.40; 1.60] | 84.4 | Ref | 80.8 |
| 20–<40 mg day−1 | 8c | 147 | 1.46 [0.82; 2.10] | 82.0 | 0.33 | ||
| >40 mg day−1 | 5c | 107 | 0.72 [0.25; 1.19] | 60.2 | 0.61 | ||
Modelling dose of flavanols, (−)-epicatechin and (+)-catechin in the clinical trial treatments
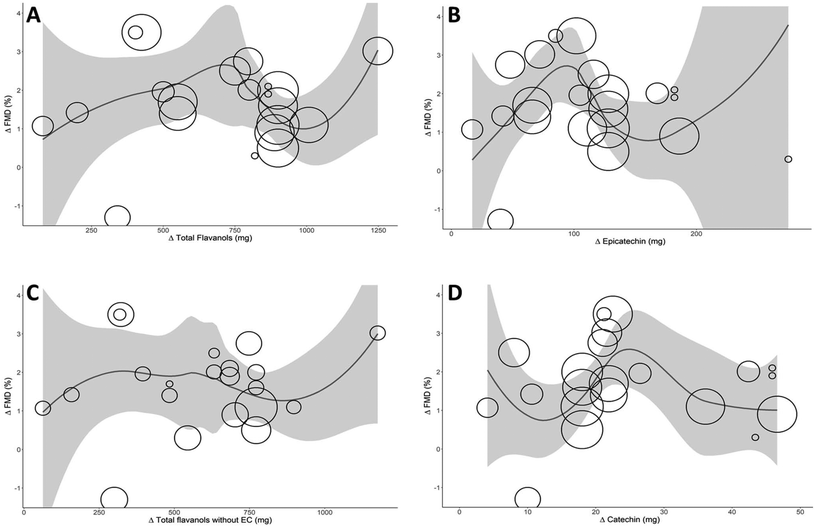
The Akaike Information Criterion (AIC) was used to define the optimal model for the relationship between the dose of total flavanols, (−)-epicatechin, (+)-catechin, and total flavanols without (−)-epicatechin and the biological effect. Fig. 3 shows the optimal models, with the degree of polynomial of 4 for total flavanols and (+)-catechin, 2 for (−)-epicatechin and 0 for total flavanols without (−)-epicatechin. An inverted U shape relationship can be seen between total flavanols, (−)-epicatechin, and (+)-catechin with FMD, with a maximum vascular effect for 710 mg total flavanols (FMD 2.5%), 95 mg (−)-epicatechin (FMD 3%) and 25 mg (+)-catechin (FMD 2.5%). A physiologically relevant effect size of 1% increase in FMD could be achieved with doses ranging from 150 to 1000 mg of total flavanols, 40 to 140 mg of (−)-epicatechin, or 15 to 38 mg of (+)-catechin. The curve for total flavanols without (−)-epicatechin depicted a different shape, suggesting that (−)-epicatechin content could be responsible for an important part of the relationship with FMD. Altogether, the results indicate that the (−)-epicatechin dose in the different interventional products plausibly drives the biological effect over the vasculature. Other ingredients also present in the treatments, such as theobromine or carbohydrates, could not be plotted since insufficient data points were available or there was no specific relationship.Sensitivity analyses and publication bias
Sensitivity analyses with fixed-effects models did not differ significantly from those observed in random-effects models (data not shown). Removing studies individually did not substantially change the differences in effect on FMD, with effect estimates ranging from 0.96% (95% CI: 0.69, 1.24%) by removing Farouque 2006A22 to 1.14% (95% CI: 0.82, 1.46%) by removing Mogollon 2013B29 (see ESI Fig. 1†). Egger's test (p = 0.08), Begg's test (p = 0.03), and the funnel plot indicated that the meta-analysis may be affected by publication bias (see ESI Fig. 2†). Overall risk of bias for each of the publications can be seen in ESI Table 1.†Discussion
The aim of this investigation was to assess the current scientific evidence on the relationship between cocoa flavanols and endothelial function, measured by FMD, and to evaluate the potential main bioactive and optimal dose from cocoa/chocolate to affect endothelial function. Our meta-analysis included 15 clinical trials, 18 intervention arms with 730 participants using interventions of at least 7 days with cocoa drink, chocolate, or both. The results showed an FMD improvement of 1.08% as a measurement of vascular endothelial function. These findings support a previous meta-analysis that described an overall effect of 1.34% FMD (CI: 1.00%, 1.68%) for studies longer than 2 weeks (9 clinical trials with 545 participants).3Brachial FMD has been inversely associated with future cardiovascular events.34–36 Meta-analyses have suggested a 13% risk reduction of cardiovascular events with a 1% increase of FMD.37,38 Hence, the improvement of FMD values observed in our meta-analysis supports the notion that chronic consumption of cocoa-derived products could decrease the overall risk of cardiovascular diseases (CVD). Importantly, there is a stronger association between FMD and CVD risk for diseased populations than for asymptomatic populations.38 Therefore, even small increases in FMD could be relevant for diseased populations.
A further subgroup analysis with different levels of total flavanols, (−)-epicatechin or (+)-catechin in the intervention treatments showed significantly higher FMD improvement with treatments containing between 50 to 150 mg of (−)-epicatechin. Interestingly, interventions with lower or higher doses of (−)-epicatechin showed a lesser effect on improving endothelial function. Modelling the dose of flavanols (−)-epicatechin and (+)-catechin in the trial treatments depicted an inverted U shape with maximum effect observed at 710 mg total flavanols, 95 mg (−)-epicatechin and 25 mg (+)-catechin. It is worth noting that (−)-epicatechin and (+)-catechin levels are correlated in the test treatments used in the analysed studies (r = 0.78). Therefore, we cannot infer the individual impact of each of them on improving endothelial function.
Acute clinical trials demonstrating improvement in FMD outcomes with other high-polyphenol-containing foods described an inverted U shape relationship similar to that observed with cocoa polyphenols in this analysis. For instance, single consumption of several doses of blueberry drinks (from freeze-dried powder) showed a dose response with a maximum effect at 766 mg of polyphenols.39 Higher doses containing 1278 or 1791 mg of polyphenols did not show a higher impact on FMD values. Another study with different doses of cranberry juice demonstrated an improvement on FMD.40 An inverted U shape was observed with the dose containing 1238 mg of total polyphenols being the most effective.
The idea that there is an optimal dose range of bioactives that would result in an optimal and physiologically relevant health effect could have large consequences for future dietary recommendations. In addition to the notion that higher concentrations do not always bring more benefits, the seasonal variation and the influence of processing on the polyphenol content in flavanol-rich foods could have significant impact on the health benefits that may be expected from the consumption of these foods. It is all the most important since, contrary to micronutrients (e.g. vitamins and minerals), these bioactives are not stored within the human body.
Our meta-analysis has some inherent limitations. Firstly, although 15 studies (with 18 study arms) were included, there is high variability in the health status of the participants as well as in the duration of the treatments (from 7 to 84 days). Heterogeneity in treatment duration and other study characteristics was investigated through subgroup analyses. However, due to varying definitions of health status used in individual studies (e.g. diabetic, hypertensive, overweight, etc.), it is impossible to conduct subgroup analyses by health status except via BMI categories. Furthermore, a number of these studies did not describe the specific flavanol levels (i.e (−)-epicatechin or (+)-catechin) in the treatments. Consequently, the levels of flavanols in these cases had to be estimated from other publications. High heterogeneity was also found in the data analysed (I2 = 80%). Subgroup analysis did not show a significant decrease in heterogeneity except for a few subgroups. Therefore, the high variability in trial characteristics, the need to estimate the levels of specific flavanols in treated groups, and the heterogeneity of primary data used, could reduce the confidence of recommendations about the effect of consuming cocoa-derived products to improve the vascular endothelial function.
In summary, our meta-analysis of randomized clinical trials confirmed previous findings that consumption of flavanol-rich foods such as cocoa drinks or chocolate could significantly improve endothelial function. In addition, the modelling of total flavanols, (−)-epicatechin and (+)-catechin content in the treatments as a function of the improvement on FMD suggests a limited range of optimal dose. However, further clinical trials, particularly with a wide range of doses, are warranted to confirm the effective dose of flavanols with optimal cardiovascular-related benefits.
Funding
This work was supported financially by Nestec Ltd, a subsidiary of Nestlé Ltd.Author contributions
LAG, YS and DZ co-designed the scope of the review and performed the literature search. YS and AdC performed the meta-analysis and subgroup analyses of the data. LAG and YS drafted the article. All authors reviewed, edited and approved the final version of the manuscript for submission.Data availability
All data generated during this study are included in the published article (and its ESI†).Abbreviations
| FMD | Flow-mediated dilation |
| RCTs | Randomized clinical trials |
| SD | Standard deviations |
| SEs | Standard errors |
| CIs | Confidence intervals |
| AIC | Akaike information criterion |
| CVD | Cardiovascular diseases |
Conflicts of interest
YS, AdC and LAG are employees of the Nestlé Research Centre, Singapore. DZ is an employee of Nestlé Research in Switzerland.Acknowledgements
Editorial support for the manuscript was obtained from Rosalind McKinnon at RAPID Consulting GmbH (Laufen, Switzerland) and funded by Nestlé.References
- X. Lin, I. Zhang, A. Li, J. E. Manson, H. D. Sesso, L. Wang and S. Liu, Cocoa Flavanol Intake and Biomarkers for Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, J. Nutr., 2016, 146, 2325–2333 CrossRef CAS PubMed.
- A. Buitrago-Lopez, J. Sanderson, L. Johnson, S. Warnakula, A. Wood, E. Di Angelantonio and O. H. Franco, Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis, Br. Med. J., 2011, 343, d4488 CrossRef PubMed.
- L. Hooper, C. Kay, A. Abdelhamid, P. A. Kroon, J. S. Cohn, E. B. Rimm and A. Cassidy, Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials, Am. J. Clin. Nutr., 2012, 95, 740–751 CrossRef CAS PubMed.
- L. Hooper, P. A. Kroon, E. B. Rimm, J. S. Cohn, I. Harvey, K. A. Le Cornu, J. J. Ryder, W. L. Hall and A. Cassidy, Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials, Am. J. Clin. Nutr., 2008, 88, 38–50 CrossRef CAS PubMed.
- D. Mozaffarian, Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review, Circulation, 2016, 133, 187–225 CrossRef CAS PubMed.
- J. I. Ottaviani, C. Heiss, J. P. E. Spencer, M. Kelm and H. Schroeter, Recommending flavanols and procyanidins for cardiovascular health: Revisited, Mol. Aspects Med., 2018, 61, 63–75 CrossRef CAS PubMed.
- J. I. Ottaviani, C. Kwik-Uribe, C. L. Keen and H. Schroeter, Intake of dietary procyanidins does not contribute to the pool of circulating flavanols in humans, Am. J. Clin. Nutr., 2012, 95, 851–858 CrossRef CAS PubMed.
- J. I. Ottaviani, G. Borges, T. Y. Momma, J. P. Spencer, C. L. Keen, A. Crozier and H. Schroeter, The metabolome of [2-(14)C](-)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives, Sci. Rep., 2016, 6, 29034 CrossRef CAS PubMed.
- C. E. Mills, A. Flury, C. Marmet, L. Poquet, S. F. Rimoldi, C. Sartori, E. Rexhaj, R. Brenner, Y. Allemann, D. Zimmermann, G. R. Gibson, D. S. Mottram, M. J. Oruna-Concha, L. Actis-Goretta and J. P. Spencer, Mediation of coffee-induced improvements in human vascular function by chlorogenic acids and its metabolites: Two randomized, controlled, crossover intervention trials, Clin. Nutr., 2017, 36(6), 1520–1529 CrossRef CAS PubMed.
- H. Schroeter, C. Heiss, J. Balzer, P. Kleinbongard, C. L. Keen, N. K. Hollenberg, H. Sies, C. Kwik-Uribe, H. H. Schmitz and M. Kelm, (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 1024–1029 CrossRef CAS PubMed.
- J. I. Dower, J. M. Geleijnse, L. Gijsbers, P. L. Zock, D. Kromhout and P. C. Hollman, Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: a randomized, double-blind, placebo-controlled, crossover trial, Am. J. Clin. Nutr., 2015, 101, 914–921 CrossRef CAS PubMed.
- J. P. Higgins, D. G. Altman, P. C. Gotzsche, P. Juni, D. Moher, A. D. Oxman, J. Savovic, K. F. Schulz, L. Weeks, J. A. Sterne, G. Cochrane Bias Methods and G. Cochrane Statistical Methods, The Cochrane Collaboration's tool for assessing risk of bias in randomised trials, Br. Med. J., 2011, 343, d5928 CrossRef PubMed.
- F. Curtin, D. Elbourne and D. G. Altman, Meta-analysis combining parallel and cross-over clinical trials. III: The issue of carry-over, Stat. Med., 2002, 21, 2161–2173 CrossRef PubMed.
- J. P. Higgins and S. G. Thompson, Quantifying heterogeneity in a meta-analysis, Stat. Med., 2002, 21, 1539–1558 CrossRef PubMed.
- H. Wickham, ggplot2: Elegant Graphics for Data Analysis, Springer-Verlag, New York, 1st edn, 2009 Search PubMed.
- J. A. Sterne and M. Egger, Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis, J. Clin. Epidemiol., 2001, 54, 1046–1055 CrossRef CAS PubMed.
- C. B. Begg and M. Mazumdar, Operating characteristics of a rank correlation test for publication bias, Biometrics, 1994, 50, 1088–1101 CrossRef CAS PubMed.
- M. Egger, G. Davey Smith, M. Schneider and C. Minder, Bias in meta-analysis detected by a simple, graphical test, Br. Med. J., 1997, 315, 629–634 CrossRef CAS PubMed.
- J. Balzer, T. Rassaf, C. Heiss, P. Kleinbongard, T. Lauer, M. Merx, N. Heussen, H. B. Gross, C. L. Keen, H. Schroeter and M. Kelm, Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial, J. Am. Coll. Cardiol., 2008, 51, 2141–2149 CrossRef CAS PubMed.
- K. Davison, A. M. Coates, J. D. Buckley and P. R. Howe, Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects, Int. J. Obes., 2008, 32, 1289–1296 CrossRef CAS PubMed.
- D. Esser, M. Mars, E. Oosterink, A. Stalmach, M. Muller and L. A. Afman, Dark chocolate consumption improves leukocyte adhesion factors and vascular function in overweight men, FASEB J., 2014, 28, 1464–1473 CrossRef CAS PubMed.
- H. M. Farouque, M. Leung, S. A. Hope, M. Baldi, C. Schechter, J. D. Cameron and I. T. Meredith, Acute and chronic effects of flavanol-rich cocoa on vascular function in subjects with coronary artery disease: a randomized double-blind placebo-controlled study, Clin. Sci., 2006, 111, 71–80 CrossRef CAS PubMed.
- A. J. Flammer, I. Sudano, M. Wolfrum, R. Thomas, F. Enseleit, D. Periat, P. Kaiser, A. Hirt, M. Hermann, M. Serafini, A. Leveques, T. F. Luscher, F. Ruschitzka, G. Noll and R. Corti, Cardiovascular effects of flavanol-rich chocolate in patients with heart failure, Eur. Heart J., 2012, 33, 2172–2180 CrossRef CAS PubMed.
- D. Grassi, S. Necozione, C. Lippi, G. Croce, L. Valeri, P. Pasqualetti, G. Desideri, J. B. Blumberg and C. Ferri, Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives, Hypertension, 2005, 46, 398–405 CrossRef CAS PubMed.
- D. Grassi, G. Desideri, S. Necozione, C. Lippi, R. Casale, G. Properzi, J. B. Blumberg and C. Ferri, Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate, J. Nutr., 2008, 138, 1671–1676 CrossRef CAS PubMed.
- D. Grassi, G. Desideri, S. Necozione, P. di Giosia, R. Barnabei, L. Allegaert, H. Bernaert and C. Ferri, Cocoa consumption dose-dependently improves flow-mediated dilation and arterial stiffness decreasing blood pressure in healthy individuals, J. Hypertens., 2015, 33, 294–303 CrossRef CAS PubMed.
- C. Heiss, S. Jahn, M. Taylor, W. M. Real, F. S. Angeli, M. L. Wong, N. Amabile, M. Prasad, T. Rassaf, J. I. Ottaviani, S. Mihardja, C. L. Keen, M. L. Springer, A. Boyle, W. Grossman, S. A. Glantz, H. Schroeter and Y. Yeghiazarians, Improvement of endothelial function with dietary flavanols is associated with mobilization of circulating angiogenic cells in patients with coronary artery disease, J. Am. Coll. Cardiol., 2010, 56, 218–224 CrossRef CAS PubMed.
- C. Heiss, R. Sansone, H. Karimi, M. Krabbe, D. Schuler, A. Rodriguez-Mateos, T. Kraemer, M. M. Cortese-Krott, G. G. Kuhnle, J. P. Spencer, H. Schroeter, M. W. Merx, M. Kelm and E. U. t. F. P. Flaviola Consortium, Impact of cocoa flavanol intake on age-dependent vascular stiffness in healthy men: a randomized, controlled, double-masked trial, Age, 2015, 37, 9794 CrossRef PubMed.
- J. A. Mogollon, E. Bujold, S. Lemieux, M. Bourdages, C. Blanchet, L. Bazinet, C. Couillard, M. Noel and S. Dodin, Blood pressure and endothelial function in healthy, pregnant women after acute and daily consumption of flavanol-rich chocolate: a pilot, randomized controlled trial, Nutr. J., 2013, 12, 41 CrossRef CAS PubMed.
- V. Y. Njike, Z. Faridi, K. Shuval, S. Dutta, C. D. Kay, S. G. West, P. M. Kris-Etherton and D. L. Katz, Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults, Int. J. Cardiol., 2011, 149, 83–88 CrossRef PubMed.
- R. Sansone, A. Rodriguez-Mateos, J. Heuel, D. Falk, D. Schuler, R. Wagstaff, G. G. Kuhnle, J. P. Spencer, H. Schroeter, M. W. Merx, M. Kelm, C. Heiss and E. U. t. F. P. Flaviola Consortium, Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: a randomised, controlled, double-masked trial: the Flaviola Health Study, Br. J. Nutr., 2015, 114, 1246–1255 CrossRef CAS PubMed.
- J. F. Wang-Polagruto, A. C. Villablanca, J. A. Polagruto, L. Lee, R. R. Holt, H. R. Schrader, J. L. Ensunsa, F. M. Steinberg, H. H. Schmitz and C. L. Keen, Chronic consumption of flavanol-rich cocoa improves endothelial function and decreases vascular cell adhesion molecule in hypercholesterolemic postmenopausal women, J. Cardiovasc. Pharmacol., 2006, 47(Suppl 2), S177–S186 CrossRef CAS PubMed , discussion S206-9.
- T. Rassaf, C. Rammos, U. B. Hendgen-Cotta, C. Heiss, W. Kleophas, F. Dellanna, J. Floege, G. R. Hetzel and M. Kelm, Vasculoprotective Effects of Dietary Cocoa Flavanols in Patients on Hemodialysis: A Double-Blind, Randomized, Placebo-Controlled Trial, Clin. J. Am. Soc. Nephrol., 2016, 11, 108–118 CrossRef CAS PubMed.
- M. L. Muiesan, M. Salvetti, A. Paini, C. Monteduro, G. Galbassini, P. Poisa, E. Porteri, C. Agabiti-Rosei, V. Paderno, E. Belotti, D. Rizzoni, M. Castellano and E. Agabiti-Rosei, Prognostic role of flow-mediated dilatation of the brachial artery in hypertensive patients, J. Hypertens., 2008, 26, 1612–1618 CrossRef CAS PubMed.
- G. Brevetti, A. Silvestro, V. Schiano and M. Chiariello, Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index, Circulation, 2003, 108, 2093–2098 CrossRef PubMed.
- S. D. Katz, K. Hryniewicz, I. Hriljac, K. Balidemaj, C. Dimayuga, A. Hudaihed and A. Yasskiy, Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure, Circulation, 2005, 111, 310–314 CrossRef PubMed.
- Y. Inaba, J. A. Chen and S. R. Bergmann, Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis, Int. J. Cardiovasc. Imaging, 2010, 26, 631–640 CrossRef PubMed.
- R. T. Ras, M. T. Streppel, R. Draijer and P. L. Zock, Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis, Int. J. Cardiol., 2013, 168, 344–351 CrossRef PubMed.
- A. Rodriguez-Mateos, C. Rendeiro, T. Bergillos-Meca, S. Tabatabaee, T. W. George, C. Heiss and J. P. Spencer, Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity, Am. J. Clin. Nutr., 2013, 98, 1179–1191 CrossRef CAS PubMed.
- A. Rodriguez-Mateos, R. P. Feliciano, A. Boeres, T. Weber, C. N. Dos Santos, M. R. Ventura and C. Heiss, Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study, Mol. Nutr. Food Res., 2016, 60, 2130–2140 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9fo01747j |
| This journal is © The Royal Society of Chemistry 2019 |