Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Characterization of bioactive compounds in commercial olive leaf extracts, and olive leaves and their infusions†
Eduardo
Medina
 *,
Concepción
Romero
*,
Concepción
Romero
 ,
Pedro
García
and
Manuel
Brenes
,
Pedro
García
and
Manuel
Brenes

Food Biotechnology Department. Instituto de la Grasa (IG-CSIC), Ctra. Utrera km 1, Building 46, 41013, Seville, Spain. E-mail: emedina@ig.csic.es
First published on 9th July 2019
Abstract
A large spectrum of beneficial health properties has been attributed to olive leaves. This study was undertaken to characterize the bioactive compounds of commercial olive leaf extracts and olive leaves and their infusions. High variability of bioactive compounds was found among commercial samples. Polyphenol was detected in a range of 44–108 g kg−1 and 7.5–250 g kg−1 for olive leaves and olive leaf extracts, respectively. The main phenol was oleuropein, representing 74–94% of total phenols. However, only 17–26% of polyphenols were diffused to the aqueous phases when olive leaf infusions were prepared. Triterpenic acids were found in a range of 26–37 g kg−1 in olive leaves, but not detected in the infusions. Hence, the absence of the latter substances and the low oleuropein diffusion in olive leaf infusions make new studies necessary to maximize the presence of these bioactive compounds in the final product.
1. Introduction
The leaves of the olive tree (Olea europaea L.), native to the Mediterranean basin, have been widely used as a folk remedy in traditional medicine. Nowadays, olive leaves (OL) can be considered as a by-product of olive farming and processing. The pruning and olive harvesting for the production of olive oil and table olives generate a considerable volume of OL, used by some industries to obtain natural products rich in bioactive compounds for food additives, dietary supplements, cosmetic and nutraceutical purposes.1–3For many centuries, olive leaves and their extracts have been associated with preservation and health. Egyptians employed OL in the mummification process as a good preservative,4 and they also have been used in folk medicine to combat fevers and other diseases, such as malaria.5
In recent times, a large spectrum of beneficial health properties in vitro and in vivo have been attributed to OL and their extracts, including an important antioxidant effect,6,7 anti-hypertensive activity,8 lower body mass and fat storage,9 and hypoglycemic effect.10 Several studies have also disclosed that olive leaf extracts (OLE) possess antimicrobial activity against certain bacteria,11 fungi12,13 and viruses.14 In addition, OLE has been related to the activity of cells involved in the inflammatory response.15,16 Furthermore, OL have been attributed with an anti-cancer inhibitive effect on tumor necrosis factor,17 anti-proliferative and pro-apoptotic properties.18
The antioxidant compounds from OL can increase the shelf life of food products by retarding the process of lipid peroxidation.19,20 Therefore, olive leaf extracts have been investigated as an additive supplement to improve the quality and stability of meat products21,22 and vegetable oils.23,24
OL are rich in bioactive substances such as phenolic compounds, triterpenic acids and sugars.25,26 The main active phenolic constituent in OL is the bitter compound oleuropein, which can constitute up to 6–9% of leaf dry matter27 which has been intensively studied for its promising results/effects on human health.4,28–30 Hydroxytyrosol and their glucosides are other components of OL with several biological activities.6,31 In addition, the surface of OL contains a high concentration of triterpenes, especially oleanolic and maslinic acids.32,33 Both contribute to the valorization of this by-product given the promising beneficial properties attributed to them, such as a potent antimicrobial, anti-tumor, anti-inflammatory, anti-HIV effect, among other activities.10,34–36 Moreover, olive leaves contain a high concentration of sugars, especially as a good source of mannitol, a bioactive substance with beneficial properties for health. The extraction of mannitol from olive leaves with ethanol has been studied for pharmaceutical purposes.37
Nowadays, there has been a growing interest in the research, development, and commercialization of functional foods, nutraceuticals and dietary supplements from natural sources in order to promote health benefits.38 OL are increasingly important as an herbal remedy and they are commercialized in the pharmaceutical market at premium prices as intact leaves or extracts. Considering the industrial interest for bioactive substances from natural sources, this study was undertaken to characterize the bioactive compounds of several commercial OLE and dried OL, as well as their infusions. The identification by HPLC of phenolic and oleosidic compounds, triterpenic acids, sugars and the antimicrobial activity of OL products against S. aureus is the goal of this study.
2. Materials and methods
2.1. Samples and infusion preparation
Ten samples of commercial olive leaves (L1–L10), seven olive leaf extract powders (E1–E7) and four liquid olive leaf extracts (E8–E11) were purchased from different herbalists or parapharmacies all over the world (Table S1†). All samples were kept at room temperature until analysis.Commercial olive leaves (1.7 g) were placed in tea bags and infused with a cup of boiling water (240 mL) for an olive leaf infusion preparation (I1–I10). The mixture rested for five minutes before removing the bag. The infusions were performed in duplicate and preserved at −40 °C for further analysis.
2.2. Analysis of phenolic compounds
The extraction of phenolic compounds from leaves and solid leaf extract was based on the methodology reported elsewhere.26 Around 2 g of cut leaves or extract powder were mixed with 30 mL of dimethyl sulfoxide (DMSO) and homogenized with an Ultra-Turrax equipment (Ika, Breisgau, Germany). After 30 minutes of resting contact, the mixture was centrifuged at 9000g for five minutes and 0.25 mL of the supernatant was diluted with 0.5 mL of DMSO plus 0.25 mL of 0.2 mM of syringic acid in DMSO as internal standard.The analysis of phenolic compounds from olive leaf infusion and liquid leaf extracts were carried out as described elsewhere.39 Olive leaf infusions were previously acidified with phosphoric acid. An aliquot of 250 μL of infusion was mixed with 500 μL of deionized water and 250 μL of syringic acid (0.2 mM) in water as internal standard.
All samples were filtered through a 0.22 μm pore size nylon filter, and an aliquot (20 μL) was injected into the chromatograph. The chromatographic system consisted of a Waters 717 plus autosampler, a Waters 600 E pump, a Waters column heater module, and a Waters 996 photodiode array detector operated with Empower 2.0 software (Waters Inc.). A 25 cm × 4.6 mm i.d., 5 μm, Spherisob ODS-2 (Waters, Inc.) column, at a flow rate of 1 mL min−1 and a temperature of 35 °C, was used in all analyses. The separation was achieved by gradient elution using water (pH 2.5 adjusted with phosphoric acid) and methanol.40 Phenolic and oleosidic compounds were monitored at 280 and 240 nm respectively. The evaluation of each compound was performed using a regression curve with the corresponding standard. Hydroxytyrosol (Hy) and verbascoside were purchased from Extrasynthese SA (Genay, France). Tyrosol (Ty), caffeic and p-coumaric acids were purchased from Sigma Chemical Co. (St Louis, MO, USA). Hydroxytyrosol 1-O-glucoside (Hy1Glu) was quantified using the response factors of Hy. Hydroxytyrosol 4-O-glucoside (Hy4Glu), ligustroside, the dialdehydic form of decarboxymethyl elenolic acid linked to hydroxytyrosol (HyEDA), oleoside, secoxyloganin, secologanoside and oleoside 11-methyl ester were obtained by semi-preparative HPLC.39,41 Samples were analyzed in duplicate.
2.3. Analysis of triterpenic acids
Triterpenic acids of olive leaves and solid olive leaf extracts were analyzed as described by Romero et al.33 Olive leaves were triturated in an Ultra-turrax to obtain a homogenized powder. Olive leaf powder or extract (0.5 g) was mixed in a 10 mL centrifuge tube with 4 mL methanol–ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and vortexed for one minute, centrifuged at 9000g for five minutes at 20 °C, and the solvent was separated from the solid phase. This step was repeated six times, and the pooled solvent extract was vacuum evaporated. The residue was dissolved in 2 mL methanol, which was filtered through a 0.22 μm pore size nylon filter and an aliquot (20 μL) was injected into the liquid chromatograph.
1, v/v) and vortexed for one minute, centrifuged at 9000g for five minutes at 20 °C, and the solvent was separated from the solid phase. This step was repeated six times, and the pooled solvent extract was vacuum evaporated. The residue was dissolved in 2 mL methanol, which was filtered through a 0.22 μm pore size nylon filter and an aliquot (20 μL) was injected into the liquid chromatograph.
The extraction of triterpenic acids from olive leaf infusion and liquid leaf extract was performed using ethyl acetate as described by Romero et al.32 Samples (0.8 mL) were mixed with 2 mL of solvent, vortexed for one minute, centrifuged at 9000g for five minutes and the ethyl acetate was separated from the liquid sample. This step was repeated six times. Subsequently, the pooled solvent extract was vacuum evaporated and the residue dissolved in 0.8 mL of methanol, which was filtered through a 0.22 μm pore size nylon filter, and an aliquot (20 μL) was injected into the liquid chromatograph. The chromatographic system and column were the same as those used for the phenolic compound analysis. The mobile phase (methanol-acidified water with phosphoric acid at pH 3.0; 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, v/v) was delivered to the column at a flow rate of 0.8 mL min−1. Oleanolic and maslinic acids were monitored at 210 nm and quantified using external standards (Sigma).
8, v/v) was delivered to the column at a flow rate of 0.8 mL min−1. Oleanolic and maslinic acids were monitored at 210 nm and quantified using external standards (Sigma).
2.4. Analysis of sugars
The concentrations of reducing sugars were determined by HPLC according to the methodology described by Romero et al.26 Briefly, homogenized olive leaves or solid leaf extracts (1 g) were mixed with 20 mL of boiling water and vortexed for one minute, kept in an ultrasonic bath for three minutes, vortexed again for one minute, and the mixture was centrifuged at 9000g for five minutes. The mixture was filtered through filter paper using a vacuum, and another 20 mL of hot water were added and filtered again. The filtrate was then transferred to a 50 mL volumetric flask containing 2 mL of sorbitol as internal standard (7.5%, w/v) and made up to volume. The solution was kept at 5 °C for 24 hours to remove lipids and subsequently filtered through a 0.22 μm pore size nylon filter. Two milliliters of the clarified liquid were put into contact with 1 g of the acidic resin Amberlite IR-120 and 1 g of the basic resin Amberlite IRA-93. Likewise, 0.5 mL of a sample of olive leaf infusion and aqueous extract were mixed with 1.5 mL of sorbitol (0.5%) before the resins treatment. Samples were shaken occasionally for 30 minutes. Then, 1 mL of the solution was centrifuged at 9000g for three minutes and filtered through a 0.22 μm pore size nylon filter. An aliquot of 20 μL was injected into the chromatograph. The HPLC system consisted of a Waters 2695 Alliance with a pump and autosampler included; the detection was performed with a Waters 410 refractive index detector. A Rezex RCM-Monosaccharide Ca+ (8%) column (300 × 7.8 mm i.d., Phenomenex) held at 85 °C and deionized water as eluent at 0.6 mL min−1 were used as described by Medina et al.392.5. Antimicrobial assays
A cocktail of strains of Staphylococcus aureus (CECT 86, 240, 86 and 975) was chosen for testing the antimicrobial effect of the olive leaf infusions and leaf extracts as they are commonly used in antimicrobial testing.42 Bacterial strains were obtained from the Spanish Type Culture Collection (Burjasot, Valencia, Spain) and were cultured in nutrient broth prepared with 5 g L−1 of Lab-Lemco powder (Oxoid), 10 g L−1 of neutralized bacteriological peptone (Oxoid), 5 g L−1 of NaCl and 15 g L−1 of agar for solid medium (pH 7.2). Every target strain was cultured in a nutrient broth from the frozen stock, before testing, and overnight cultures were used for inoculum preparation.The olive leaves L8 and the leaf extract E8 were selected for the antimicrobial test. The commercial olive leaves L8 were prepared in three different crushing degrees: intact leaves (normal), crushed under a blender (blender) and as a powder by an Ultra-turrax equipment (ultra). Finally, they were infused as described above (section 2.1) and the aqueous phases were inoculated for antimicrobial testing. E8 and its dilution with saline solution was tested at concentrations of 100, 50, 25 and 10%. Saline solution (0.85 g per 100 mL) was selected as positive control. Five milliliters of the sample were inoculated and mixed with 50 μL of an overnight culture of S. aureus diluted with saline solution to obtain an initial population between 6.11 and 6.37 log CFU mL−1.
The mixture was incubated at room temperature for one and 24 hours. After treatment, culturable survivors were determined by plating these mixtures or corresponding decimal dilutions (0.1% peptone water) on nutrient agar media plating with a Spiral Plater (Don Whitley Sci. Ltd, model WASP 2, Shipley, UK). Colonies were enumerated with an automated counter (Countermat, IUL Instruments, Barcelona, Spain). The antimicrobial test was performed in duplicate.
2.6. Panel test
Infusions prepared with olive leaves L8 with the three degrees of crushing (control, blender and ultra-turrax) were organoleptically evaluated by a 13 member non-trained panel in a standardized testing room. The objective was to determine the bitterness and other relevant attributes detected by panelists in order to understand the acceptability of consumption.2.7. Statistical analysis
Statistica software 10.0 (StatSoft, Inc., Tulsa, OK, USA) was used for data analysis. Data were expressed as mean values ± standard deviation. Statistical comparisons of the mean values for each experiment were performed by one-way analysis of variance followed by the Duncan's multiple range test and the differences were considered significant when p < 0.05.3. Results and discussion
The phenolic composition of commercial OL and OLE samples is shown in Table 1. The total polyphenols concentration in OL showed high variability in a range of 44.79–108.27 g kg−1. Among polyphenols, oleuropein was clearly the major compound representing more than 88–94% of total phenolic compounds. Ligustroside, hydroxytyrosol 1-glucoside and hydroxytyrosol 4-glucoside were also present at significant concentrations, ranging between 2–7%, 2–1% and 0.5–2.5% of total phenolic compounds, respectively. Verbascoside and caffeic acid were found in minor concentrations. It must be noted that these results are in line with those obtained for other authors.26,43,44 In the same way, the variability was highly remarkable for commercial OLE with a wide range for total phenols, between 7.50 and 249.81 g kg−1. Again, oleuropein was the main phenol, representing more than 84–90% of total polyphenols.| Sample | Hy4Glu | Hy1Glu | Verbascoside | Caffeic acid | p-Cumaric acid | HyEDA | Oleuropein | Ligustroside | Total polyphenols |
|---|---|---|---|---|---|---|---|---|---|
| L1 | 0.74 (0.03)ab | 0.95 (0.03)d | 0.70 (0.03)ab | 0.04 (0.01)d | ND | ND | 78.48 (1.55)ab | 2.52 (0.53)a | 83.42 (2.11)a |
| L2 | 1.15 (0.21)c | 1.27 (0.23)cb | 1.78 (0.31)e | 0.06 (0.02)abc | ND | ND | 51.89 (6.31)e | 2.65 (0.29)ab | 58.80 (7.35)d |
| L3 | 0.26 (0.02)f | 0.59 (0.14)a | 0.51 (0.16)ac | 0.07 (0.02)bc | ND | ND | 40.30 (9.12)d | 3.05 (0.29)abc | 44.79 (9.72)c |
| L4 | 2.22 (0.22)e | 1.19 (0.01)b | 1.19 (0.02)d | 0.07 (0.01)c | ND | ND | 91.06 (0.75)c | 3.76 (1.45)abc | 99.48 (0.47)be |
| L5 | 0.90 (0.20)abc | 0.58 (0.07)a | 0.71 (0.05)ab | 0.05 (0.01)abd | ND | ND | 75.85 (5.88)a | 3.59 (0.23)abc | 81.69 (6.43)a |
| L6 | 0.63 (0.07)a | 1.60 (0.12)e | 0.36 (0.00)c | 0.04 (0.00)ad | ND | ND | 71.50 (3.15)a | 2.95 (0.19)abc | 77.07 (3.14)a |
| L7 | 1.96 (0.31)de | 1.26 (0.04)cb | 1.42 (0.01)d | 0.06 (0.00)abc | ND | ND | 79.83 (3.00)ab | 3.85 (0.33)abc | 88.38 (3.68)ab |
| L8 | 1.13 (0.12)bc | 0.43 (0.01)a | 0.71 (0.05)ab | 0.06 (0.00)abc | ND | ND | 88.92 (4.36)bc | 4.16 (0.12)c | 95.41 (4.41)b |
| L9 | 1.69 (0.14)d | 0.94 (0.02)d | 2.31 (0.19)f | 0.10 (0.00)f | ND | ND | 99.30 (1.93)c | 3.94 (0.30)cd | 108.28 (1.97)e |
| L10 | 0.87 (0.07)abc | 1.44 (0.04)ec | 0.88 (0.02)b | 0.05 (0.00)abcd | ND | ND | 49.75 (0.22)de | 1.18 (0.23)d | 54.17 (0.02)cd |
| E1 | 1.07 (0.26)a | 2.15 (0.17)a | 0.84 (0.03)ab | 0.07 (0.00)bcd | 0.04 (0.01)ab | 0.08 (0.15)a | 62.82 (1.87)d | 4.53 (1.43)f | 72.27 (3.91)d |
| E2 | 3.76 (0.26)b | 3.49 (0.18)bcd | 3.29 (0.14)def | 0.13 (0.02)f | 0.04 (0.00)ab | ND | 143.77 (3.34)ab | 9.11 (0.84)a | 163.60 (3.85)ab |
| E3 | 9.07 (3.28)c | 10.51 (1.31)f | 1.89 (3.19)bcd | 0.09 (0.00)de | 0.19 (0.04)e | ND | 183.32 (9.70)e | 11.71 (1.06)c | 216.79 (6.54)e |
| E4 | 1.72 (0.81)ab | 2.63 (0.19)abc | 4.56 (0.11)f | 0.08 (0.00)cde | 0.10 (0.02)cd | 2.10 (0.27)b | 129.62 (3.08)ab | 8.93 (0.47)a | 149.74 (3.58)ab |
| E5 | 7.68 (0.33)c | 3.00 (0.43)abc | 2.68 (0.24)cde | 0.15 (0.02)f | 0.04 (0.00)ab | ND | 117.94 (2.12)a | 7.91 (0.03)a | 139.41 (1.76)a |
| E6 | 9.07 (1.27)c | 4.50 (0.41)d | 10.20 (1.19)g | 0.22 (0.02)g | 0.11 (0.00)d | ND | 213.80 (1.68)f | 11.91 (0.03)c | 249.81 (1.03)f |
| E7 | 0.76 (0.02)a | 2.51 (0.02)ab | 3.56 (0.21)ef | 0.10 (0.02)e | 0.07 (0.00)bc | 2.74 (0.05)c | 191.40 (2.45)ef | 8.92 (0.05)a | 210.05 (2.69)e |
| E8 | 0.57 (0.03)a | 0.94 (0.21)e | 0.03 (0.01)a | 0.02 (0.00)a | ND | 1.05 (0.07)d | 8.23 (0.65)c | 0.22 (0.08)b | 11.08 (0.87)c |
| E9 | 2.77 (0.64)ab | 3.73 (0.12)cd | 1.73 (0.13)bc | 0.06 (0.01)bc | ND | ND | 150.59 (35.39b) | 7.96 (0.64)a | 166.84 (33.39)b |
| E10 | 0.22 (0.04)a | 0.13 (0.05)e | 0.08 (0.02)a | 0.01 (0.00)a | ND | ND | 6.74 (1.62)c | 0.31 (0.16)b | 7.50 (1.53)c |
| E11 | 1.27 (0.13)ab | 3.19 (0.52)abc | 1.40 (0.07)abc | 0.04 (0.00)ab | 0.01 (0.01)a | 0.33 (0.02)e | 40.95 (6.32)d | 1.77 (0.38)d | 48.96 (6.65)d |
This variability in the phenolic composition can be explained by the interaction of the type of cultivar, climate, and the geographic production zone.45 The harvesting time can also be influential since OL reach their maximum phenolic content in the cold season around December.26,43,44 In addition, the elaboration process, the extraction process and problems with the stability of commercial products contribute to this large heterogeneity in the phenolic composition.46
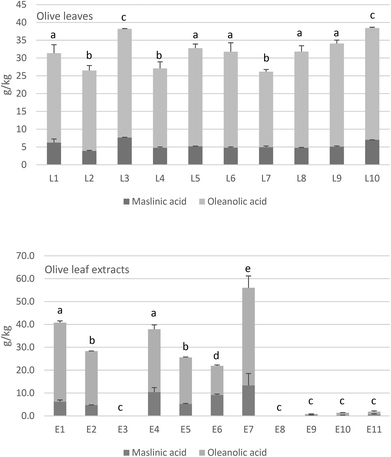
Commercial OL also contained a high amount of triterpenic acids that ranged between 25 and 35 g kg−1 as shown in Fig. 1. The major triterpene was oleanolic acid, which represents up to 79–89%, followed by maslinic acid with 14–20%, both compounds with well-known health properties. Again, the olive cultivar can influence the content of triterpenic acids in the leaves,37 as well as whether it is picked from the ground or the tree.33 However, the variability of triterpenic acids (Fig. 1) was lower than found for phenolic compounds (Table 1), probably due to the higher stability of the former substances during the extraction steps than the latter.
Regarding the OLEs, we found that triterpenes were not detected or were present in low concentrations in some of the extracts (E3, E8, E9, E10, E11). Conversely, the rest of the OLEs contained high amounts of triterpenic acids, even reaching more than 50 g kg−1 in the case of E7. As commented above, the raw material determines the final content in the products, but the extraction method employed in the extract's elaboration can also be influential for a good recovery of these bioactive compounds.
Mannitol was the main sugar detected in OL and OLE samples followed by the saccharides sucrose, glucose and fructose in lower concentrations (Table 2). Variation in total sugars concentration was not remarkable for OL, but it was more marked in OLE which presented a range between 8.57–245.9 g kg−1. The results are in line with those found in Picual, Arbequina and Manzanilla olive leaves,47 but they found a higher glucose concentration dependent on the cultivar. As commented before, the cultivar and the harvesting season influence the amount of sugars in the OL.26 Olive leaves can also be a good source of mannitol as a bioactive substance since it presents beneficial properties for health, thus being very useful for pharmaceutical purposes.37
| Sample | Sucrose | Glucose | Fructose | Mannitol | Total sugars |
|---|---|---|---|---|---|
| L1 | 14.83 (1.32)a | 9.76 (0.07)e | 7.94 (0.10)a | 41.71 (3.92)de | 74.23 (5.42)b |
| L2 | 26.61 (1.40)f | 15.14 (1.43)abc | 12.47 (0.75)d | 33.75 (2.23)a | 87.97 (5.80)a |
| L3 | 11.02 (0.72)e | 16.66 (0.71)bc | 5.35 (0.20)e | 27.16 (1.35)f | 60.20 (2.98)c |
| L4 | 31.25 (0.15)c | 14.81 (1.82)ab | 8.38 (0.63)ab | 38.58 (0.89)bd | 93.02 (2.23)a |
| L5 | 20.62 (0.43)b | 13.47 (1.16)a | 8.67 (0.25)ab | 44.56 (1.56)ce | 87.31 (2.90)a |
| L6 | 14.02 (0.31)a | 17.47 (0.22)cd | 8.84 (0.06)abc | 35.31 (0.41)ab | 75.65 (0.06)b |
| L7 | 30.11 (0.07)c | 13.40 (1.02)a | 9.25 (0.06)bc | 34.89 (0.06)ab | 87.64 (1.09)a |
| L8 | 20.44 (0.50)b | 14.84 (0.74)ab | 8.64 (0.26)ab | 48.08 (0.07)c | 92.00 (0.08)a |
| L9 | 37.66 (1.06)g | 17.45 (0.91)cd | 9.75 (0.75)c | 47.13 (0.28)c | 112.00 (3.00)d |
| L10 | 6.37 (0.14)d | 19.40 (1.17)d | 12.38 (0.22)d | 34.91 (0.71)ab | 73.06 (1.53)b |
| E1 | 9.58 (0.28)d | 11.33 (13.12)bc | 9.77 (0.02)d | 40.13 (0.91)d | 70.81 (11.92)b |
| E2 | 18.27 (0.02)c | 13.74 (1.43)c | 6.14 (1.24)c | 26.03 (0.03)c | 64.19 (2.62)b |
| E3 | 55.74 (0.60)b | 34.89 (2.81)de | 14.69 (3.47)b | 82.40 (0.78)g | 187.71 (6.10)f |
| E4 | 21.64 (1.53)c | 16.23 (2.53)c | 5.88 (1.49)c | 15.99 (2.55)b | 59.74 (8.09)b |
| E5 | 38.29 (4.98)e | 28.96 (0.88)d | 13.78 (1.08)b | 68.51 (0.62)f | 149.53 (3.63)c |
| E6 | 61.25 (0.47)b | 50.91 (6.00)f | 25.88 (1.89)e | 107.92 (1.51)h | 245.95 (8.93)f |
| E7 | 59.08 (9.69)b | 40.11 (2.95)e | 12.10 (1.12)bd | 54.60 (1.27)e | 165.90 (12.78)d |
| E9 | 3.02 (0.00)a | 10.44 (0.00)ab | 0.64 (0.00)a | 15.95 (0.00)a | 15.03 (21.26)a |
| E8 | 1.39 (0.06)a | 1.40 (0.26)a | 0.53 (0.00)a | 5.25 (0.21)a | 8.57 (0.12)a |
| E10 | 2.95 (0.37)a | 3.50 (0.32)a | 0.68 (0.03)a | 5.41 (2.29)a | 12.54 (3.01)a |
| E11 | 12.62 (5.08)a | 7.41 (2.16)ab | 2.97 (0.82)a | 21.92 (8.30)a | 44.92 (16.36)a |
The concentration of phenolic compounds and sugars in the aqueous phase of OL infusions is shown in Table 3. First, it must be highlighted that only 12–27% of the initial polyphenol concentration found in OL was diffused to the aqueous phase, with oleuropein being the more abundant phenol. Instead, sugar diffusion was higher. Around 41–80% of sugars in OL were diffused to the aqueous phase because of the high solubility in water of these compounds. In contrast, triterpenic acids were not detected in any of the infusions tested, due to their apolar nature.
| Infusion sample | Hy4Glu | Hy1Glu | Verbascoside | Oleuropein | Total polyphenols | Sucrose | Glucose | Fructose | Mannitol | Total sugars |
|---|---|---|---|---|---|---|---|---|---|---|
| I1 | 2.3 (1.2)b | 4.1 (0.7)abc | 1.1 (0.1)bd | 125.8 (3.2)ab | 140.6 (0.2)a | 0.0 (0.0)d | 49.6 (24.2)a | 142.6 (10.6)ab | 98.3 (16.8)abc | 290.5 (30.5)ab |
| I2 | 3.7 (0.4)a | 5.2 (0.1)abd | 2.5 (0.2)c | 85.5 (4.9)c | 104.4 (4.9)b | 50.1 (0.3)ac | 60.9 (10.2)abc | 156.9 (9.3)ab | 92.7 (9.6)ab | 360.5 (8.4)ab |
| I3 | ND | 1.8 (0.1)c | ND | 33.7 (4.9)e | 37.7 (5.2)c | 14.1 (19.9)bcd | 33.9 (7.6)a | 123.6 (28.7)a | 33.5 (4.6)b | 205.1 (45.5)b |
| I4 | 6.3 (0.5)cd | 4.7 (0.5)ab | 2.0 (0.6)ac | 128.1 (9.3)ab | 150.9 (11.1)a | 57.5 (0.7)a | 63.8 (0.6)abc | 123.9 (1.2)a | 92.7 (1.0)ab | 337.9 (3.5)ab |
| I5 | 3.2 (0.0)ab | 3.0 (0.5)ac | 1.4 (0.0)abc | 132.6 (2.0)ab | 149.4 (0.5)a | 45.1 (23.9)abc | 96.0 (29.2)abc | 147.9 (27.6)ab | 157.1 (84.8)ac | 446.1 (165.5)ac |
| I6 | ND | 9.3 (2.6)f | 0.6 (0.3)d | 85.9 (2.3)c | 101.1 (3.9)b | 44.0 (3.4)abc | 121.0 (52.5)bc | 189.4 (26.6)b | 79.9 (12.1)ab | 434.2 (94.6)ac |
| I7 | 5.3 (0.5)d | 5.2 (0.4)abd | 2.1 (0.0)ac | 118.8 (3.4)a | 140.9 (4.1)a | 57.7 (19.1)a | 59.4 (21.2)ab | 151.8 (13.2)ab | 117.3 (5.3)abc | 386.3 (16.4)ab |
| I8 | 2.7 (0.1)c | 2.6 (0.0)bd | 1.6 (0.0)e | 156.7 (17.4)d | 174.0 (17.8)d | 39.2 (0.1)e | 59.0 (0.6)c | 172.3 (3.2)b | 180.9 (4.5)c | 451.4 (6.5)c |
| I9 | 7.1 (0.3)ab | 6.6 (1.8)ac | 4.7 (0.9)ab | 180.0 (35.1)bd | 209.9 (34.7)a | 114.9 (34.5)abc | 126.8 (38.8)ab | 197.0 (64.6)ab | 178.9 (66.2)c | 617.6 (204.1)ac |
| I10 | 3.7 (0.8)a | 7.5 (0.5)de | 1.7 (0.1)abc | 67.4 (14.1)c | 86.4 (15.4)b | 8.3 (11.7)bd | 75.4 (30.8)abc | 143.9 (1.7)ab | 120.8 (8.2)ac | 348.3 (36.1)ab |
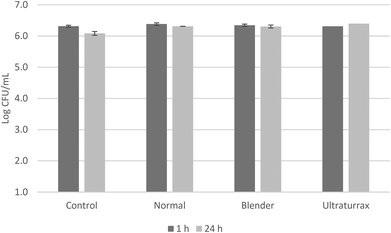
The crushing degree of the leaves could be a factor to take into account for a better diffusion of bioactive compounds from the leaves to the water phase of the infusions. For the study of this hypothesis, infusions were made with sample L8 with several crushing degrees: control without an extra crushing, crushed with a blender and with an Ultra-turrax equipment, with an oleuropein concentration of 103, 207 and 466 mg kg−1, respectively. Therefore, it was observed that the higher the degree of trituration, the greater the amount of phenols diffused into the aqueous phase during the infusion elaboration.
Oleuropein is a phenolic glucoside that confers a bitter taste to the olive infusions and consequently the higher the quantity of this compound, the greater the bitter taste. A panel test was performed with non-trained panelists in order to detect the degree of acceptance of the bitterness in the three infusions prepared with L8 with different crushing degrees. Six out of 13 panelists detected a slightly bitter taste with the infusion prepared with L8 without an extra crushing, and two panelists detected a light astringent taste. However, all of them would consume the product. It must be noted that the L8 infusion had the second highest concentration of oleuropein among the tested olive leaves (Table 3). The bitterness of the infusion with L8 crushed with a blender was more notable. All of the panelists detected the bitter taste and four out of the 13 detected the astringent sensation as well. This increase in bitterness caused 23% of panelists to say they would not consume this product. The infusion made with the ultra-turrax leaves showed a very intense bitter taste accompanied by a more pronounced astringency. The acceptability was less for this case, with 92% of panelists saying they would not consume this product.
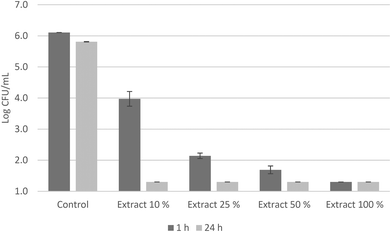
An antimicrobial test was carried out with these infusions against a cocktail of S. aureus strains (Fig. 2). None of the infusions exerted any antimicrobial activity against S. aureus after 24 hours of contact, regardless of the degree of trituration. The oleuropein concentration of 466 mg kg−1 (infusion made with ultra-turrax leaves) was not enough high to decrease the initial bacterial population. Conversely, the olive leaf extract E8 had a remarkable antimicrobial activity against the S. aureus cocktail (Fig. 3). The initial bacterial population decreased by 2 log units in one hour of contact when the extract was diluted at 10% and was more pronounced when concentrations increased. Also, after 24 hours, survivals were detected below the detection limits at all concentrations. Sudjana et al.11 also reported the antimicrobial activity of OLE against S. aureus and other bacteria as Campylobacter jejuni and Helicobacter pylori and their role in regulating the composition of the gastric microbiota.
However, the olive leaf extract E8 showed a total polyphenol concentration of 11.08 g kg−1, of which 8.23 g kg−1 corresponded to oleuropein (Table 1). It is worth noting the presence of HyEDA in this extract, at a concentration of 1.05 g kg−1. This compound has been demonstrated as a powerful antimicrobial polyphenol, found in products derived from olives such as oil or table olives.48 Medina et al.49 reported a minimal bactericidal concentration (MBC) of 0.048 g kg−1 of HyEDA against S. aureus, while oleuropein did not show any bactericidal activity at a concentration as high as 10.80 g kg−1. In addition, the presence of oleoside 11-methyl ester in the extract at a concentration of 218 mg kg−1 (Table S2†) can contribute to the antimicrobial activity exerted. It has been demonstrated that this compound had bactericidal activity against lactic acid bacteria in Spanish-style green olives.50 Likewise, other olive by-products have been studied as a natural source of antimicrobial compounds and their use in the food industry, as well as preservatives and nutraceuticals with functional properties.33
The use of OLE in the food industry can contribute beneficially to a better food preservation due to its high antimicrobial properties.11–13 This natural antimicrobial activity can reduce the spoilage microbiota and increase the shelf life of the product,19 as well as from a food safety point of view by inhibiting foodborne pathogens,51,52 or providing beneficial health effects in the gut microbiota of consumers.11,30 The antimicrobial activity of OLE has been studied by several authors in order to increase the shelf life of foodstuffs, such as inhibition of lactic acid bacteria and enterobacteria in refrigerated turkeys;19 reducing S. enterica in leafy greens51 or against L. monocytogenes in cold-smoked salmon52 but no correlation was made with any particular compound.
The high variability in the concentration of bioactive compounds found for OLE (Table 1) can be due to various factors such as raw material or the extraction process among others. However, the final composition of the OLE has great importance in order to exert an antimicrobial effect. Our data indicate that the presence of oleuropein at this concentration is not enough to exert antimicrobial activity. Elsewhere, the presence of those phenolic compounds with antimicrobial activity such as HyEDA or oleoside 11-methyl ester could explain the antimicrobial properties of olive leaves.
4. Conclusions
The beneficial properties of OL have been attributed to their composition, especially to their content in phenolic compounds, triterpenic acids, and sugars. In this study, great variability in the composition of commercial OL and OLE has been observed. The raw material, cultivar, harvesting period or elaboration process have an important role in order to obtain products rich in bioactive compounds.In OL infusions, the diffusion of triterpenic acids is null and the phenolic compounds depend on the trituration degree of the leaves. Some OLE tested exerted a high antimicrobial activity against S. aureus, even when diluted. However, OL infusions did not show any activity regardless of the crushing degree. Further studies will be necessary to improve the elaboration of OL and OLE to increase their amount of bioactive compounds and their use as a potential source of antibacterial compounds for the food and pharmaceutical industry.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors wish to acknowledge Ms Alejandra Expósito for technical expertise.References
- S. Sahin and M. Bilgin, Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry, J. Sci. Food Agric., 2015, 98, 1271–1279 CrossRef.
- S. Sahin, E. Elhussein, M. Bilgin, J. M. Lorenzo, F. J. Barba and S. Roohinejad, Effect of drying method on oleuropein, total phenolic content, flavonoid content, and antioxidant activity of olive (Olea europaea) leaf, J. Food Process. Preserv., 2018, 42, e13604 CrossRef.
- S. Sahin, R. Samli, A. S. B. Tan, F. J. Barba, F. Chemat, G. Cravotto and J. M. Lorenzo, Solvent-free microwave-assisted extraction of polyphenols from olive tree leaves: antioxidant and antimicrobial properties, Molecules, 2017, 22, 1056 CrossRef.
- C. Soler-Rivas, J. C. Espin and H. J. Wichers, Oleuropein and related compounds, J. Sci. Food Agric., 2000, 80, 1013–1023 CrossRef CAS.
- D. Hanbury, On the febrifuge properties of the olive (Olea europaea L.), Pharmaceut, J. Provincial Trans., 1854, 353–354 Search PubMed.
- S. N. El and S. Karakaya, Olive tree (Olea europaea) leaves: potential beneficial effects on human health, Nutr. Rev., 2009, 67, 632–638 CrossRef.
- M. Ben Salem, H. Affes, K. Ksouda, Z. Sahnoun, K. M. Zeghal and S. Hammami, Pharmacological activities of Olea europaea leaves, J. Food Process. Preserv., 2015, 39, 3128–3136 CrossRef CAS.
- E. Susalit, M. Agus, I. Effendi, R. Tjandrawinata, D. Nofiarny, T. Perrinjaquet-Moccetti and M. Verbruggen, Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with Captopril, Phytomedicine, 2011, 18, 251–258 CrossRef CAS.
- F. Paiva-Martins, S. Barbosa, M. Silva, D. Monteiro, V. Pinheiro, J. L. Mourão, J. Fernandes, S. Rocha, L. Belo and A. Santos-Silva, The effect of olive leaf supplementation on the constituents of blood and oxidative stability of red blood cells, J. Funct. Foods, 2014, 9, 271–279 CrossRef CAS.
- L. I. Somova, F. O. Shode and M. Mipando, Cardiotonic and antidysrhythmic effects of Oleanolic and ursolic acids, methyl maslinate and uvaol, Phytomedicine, 2004, 11, 121–129 CrossRef CAS.
- A. N. Sudjana, C. D'Orazio, V. Ryan, N. Rasool, J. Ng, T. V. Riley and K. Hammer, Antimicrobial activity of commercial Olea europaea, (olive) leaf extract, Int. J. Antimicrob. Agents, 2009, 33, 461–463 CrossRef CAS PubMed.
- M. Korukluoglu, Y. Sahan and A. Yigit, Antifungal properties of olive leaf extracts and their phenolic compounds, J. Food Saf., 2008, 28, 76–87 CrossRef CAS.
- Z. Shialy, M. Zarrin, B. Sadeghi Nejad and S. Yusef Naanaie, In vitro antifungal properties of Pistacia atlantica and olive extracts on different fungal species, Curr. Med. Mycol., 2015, 1, 40–45 CrossRef CAS.
- V. Micol, N. Caturla, L. Perez-Fons, V. Mas, L. Pérez and A. Estepa, The olive leaf extract exhibits antiviral activity against viral haemorrhagic septicaemia rhabdovirus (VHSV), Antiviral Res., 2005, 66, 129–136 CrossRef CAS PubMed.
- T. Vezza, F. Algieri, A. Rodríguez-Nogales, J. Garrido-Mesa, M. P. Utrilla, N. Talhaoui, A. M. Gomez-Caravaca, A. Segura-Carretero, M. E. Rodríguez-Cabezas, G. Monteleone and J. Galvez, Immunomodulatory properties of Olea europaea leaf extract in intestinal inflammation, Mol. Nutr. Food Res., 2017, 61, 601066 CrossRef.
- T. Magrone, A. Spagnoletta, R. Salvatore, M. Magrone, F. Dentamaro, M. A. Russo, G. Difonzo, C. Summo, F. Caponio and E. Jirillo, Olive Leaf Extracts Act as Modulators of the Human Immune Response, Endocr., Metab. Immune Disord.: Drug Targets, 2018, 18, 85–93 CAS.
- S. Nishibe, Y. Han, Y. Noguchi, H. Ueda, M. Yamazaki, K. Mizutani, T. Kambara and N. Kishida, The inhibitory effects of the compounds from olive leaf on tumor necrosis factor production and on β-hexosaminidase release, J. Nat. Med., 2001, 55, 205–208 CAS.
- J. Anter, Z. Fernández-Bedmar, M. Villatoro-Pulido, S. Demyda-Peyr, M. Moreno-Millán, A. Alonso-Moraga, A. Muñoz-Serrano and M. D. Luque de Castro, A pilot study on the DNA-protective, cytotoxic, and apoptosis-inducing properties of olive-leaf extracts, Mutat. Res., 2011, 723, 165–170 CAS.
- E. Botsoglou, A. Govaris, E. Christaki and N. Botsoglou, Effect of dietary olive leaves and/or a-tocopheryl acetate supplementation on microbial growth and lipid oxidation of turkey breast fillets during refrigerated storage, Food Chem., 2010, 121, 17–22 CrossRef CAS.
- V. Gök and Y. Bor, Effect of olive leaf, blueberry and Zizyphus jujuba extracts on the quality and shelf life of meatball during storage, J. Food Agric. Environ., 2012, 10, 190–195 Search PubMed.
- J. E. Hayes, V. Stepanyan, P. Allen, M. N. O'Grady and J. P. Kerry, Effect of lutein, sesamol, ellagic acid and olive leaf extract on the quality and shelf-life stability of packaged raw minced beef patties, Meat Sci., 2010, 84, 613–620 CrossRef CAS.
- F. Aouidi, A. Okba and M. Hamdi, Valorization of functional properties of extract and powder of olive leaves in raw and cooked minced beef meat, J. Sci. Food Agric., 2017, 97, 3195–3203 CrossRef CAS.
- V. Sanchez de Medina, F. Priego-Capote and M. D. Luque de Castro, Characterization of Refined Edible Oils Enriched with Phenolic Extracts from Olive Leaves and Pomace, J. Agric. Food Chem., 2012, 60, 5866–5873 CrossRef CAS.
- A. Zribi, B. Gargouri, H. Jabeur, A. Rebai, R. Abdelhedi and M. Bouaziz, Enrichment of pan-frying refined oils with olive leaf phenolic-rich extract to extend the usage life, Eur. J. Lipid Sci. Technol., 2013, 115, 1443–1453 CrossRef CAS.
- N. A. Nunes, F. B. Pimentel, A. S. G. Costa, R. C. Alves and M. B. P. P. Oliveira, Olive by-products for functional and food applications: challenging opportunities to face environmental constraints, Innovative Food Sci. Emerging Technol., 2016, 35, 139–148 CrossRef.
- C. Romero, E. Medina, M. A. Mateo and M. Brenes, Quantification of bioactive compounds in Picual and Arbequina olive leaves and fruit, J. Sci. Food Agric., 2017, 97, 1725–1732 CrossRef CAS PubMed.
- A. Romani, S. Mulas and D. Heimler, Polyphenols and secoiridoids in raw material (Olea europaea, L. leaves) and commercial food supplements, Eur. Food Res. Technol., 2017, 243, 429–435 CrossRef CAS.
- I. Andreadou, E. K. Iliodromitis, E. Mikros, M. Constantinou, A. Agalias, P. Magiatis, A. L. Skaltsounis, E. Kamber, A. Tsantili-Kakoulidou and D. Kremastinos D, The olive constituent oleuropein exhibits anti-ischemic, antioxidative, and hypolipidemic effects in anesthetized rabbits, J. Nutr., 2006, 136, 2213–2219 CrossRef CAS.
- M. Laguerre, L. J. López Giraldo, G. Piombo, M. C. Figueroa-Espinoza, M. Pina, M. Benaissa, A. Combe, A. Rossignol Castera, J. Lecomte and P. Villeneuve, Characterization of olive-leaf phenolics by ESI-MS and evaluation of their antioxidant capacities by the CAT assay, J. Am. Oil Chem. Soc., 2009, 86, 1215–1225 CrossRef CAS.
- T. Žugčić, R. Abdelkebir, C. Alcantara, M. C. Collado, J. V. García-Pérez, A. J. Meléndez-Martínez, A. R. Jambrak, J. M. Lorenzo and F. J. Barba, From extraction of valuable compounds to health promoting benefits of olive leaves through bioaccessibility, bioavailability and impact on gut microbiota, Trends Food Sci. Technol., 2019, 83, 63–77 CrossRef.
- R. Briante, M. Patumi, S. Terenziani, E. Bismuto, F. Febbraio and R. Nucci, Olea europaea L. leaf extract and derivatives: antioxidant properties, J. Agric. Food Chem., 2002, 50, 4934–4940 CrossRef CAS.
- C. Romero, A. García, E. Medina, M. V. Ruíz-Méndez, A. de Castro and M. Brenes, Triterpenic acids in table olives, Food Chem., 2010, 118, 670–674 CrossRef CAS.
- C. Romero, E. Medina, M. A. Mateo and M. Brenes, New by-products rich in bioactive substances from the olive mill processing, J. Sci. Food Agric., 2018, 98, 225–230 CrossRef CAS.
- I. Baglin, A. C. Mitaine-Offer, N. Nour, K. Tan, C. Cavé and M. A. Lacaille-Dubois, A review of natural and modified betulinic, ursolic and echinocystic acid derivatives as potential antitumor and anti-HIV agents, Mini-Rev. Med. Chem., 2003, 3, 525–539 CrossRef CAS.
- A. Fontanay, M. Grare, J. Mayer, C. Finance and R. E. Duval, Ursolic, oleanolic and betulinic acids: antibacterial spectra and selectivity indexes, J. Ethnopharmacol., 2008, 120, 272–276 CrossRef.
- F. J. Reyes-Zurita, E. E. Rufino-Palomares, J. A. Lupiáñez and M. Cascante, Maslinic acid, a natural triterpene from Olea europaea L. induces apoptosis in HT29 human colon-cancer cells via the mitochondrial apoptotic pathway, Cancer Lett., 2009, 273, 44–54 CrossRef CAS.
- A. Guinda, J. M. Castellano, J. M. Santos-Lozano, T. Delgado-Hervás, P. Gutiérrez-Adánez and M. Rada, Determination of major bioactive compounds from olive leaf, LWT – Food Sci. Technol., 2015, 64, 431–438 CrossRef CAS.
- L. Day, R. B. Seymour, K. F. Pitts, I. Konczak and L. Lundin, Incorporation of functional ingredients into foods, Trends Food Sci. Technol., 2009, 20, 388–395 CrossRef CAS.
- E. Medina, M. Brenes, C. Romero, A. García and A. de Castro, Main antimicrobial compounds in table olives, J. Agric. Food Chem., 2007, 55, 9817–9823 CrossRef CAS.
- E. Ramírez, E. Medina, M. Brenes and C. Romero, Endogenous enzymes involved in the transformation of oleuropein in Spanish table olive varieties, J. Agric. Food Chem., 2014, 62, 9569–9575 CrossRef.
- E. Medina, C. Romero, M. Brenes, A. de Castro and A. García, Profile of antilactic acid bacteria compounds during the storage of olives which are not treated with alkali, Eur. Food Res. Technol., 2008, 228, 133–138 CrossRef CAS.
- European Standard EN 1276, Chemical disinfectants and antiseptics—quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic, and institutional areas, BSI, London, 1997.
- S. Sahin, N. S. A. Malik, J. L. Perez and J. E. Brockington, Seasonal changes of individual phenolic compounds in leaves of twenty olive cultivars grown in Texas, J. Agric. Sci. Technol., 2012, 2, 242–247 Search PubMed.
- N. Talhaoui, A. M. Gómez-Caravaca, C. Roldán, L. León, R. De la Rosa, A. Fernandez-Gutiérrez and A. Segura-Carretero, Chemometric Analysis for the Evaluation of Phenolic Patterns in Olive Leaves from Six Cultivars at Different Growth Stages, J. Agric. Food Chem., 2015, 63, 1722–1729 CrossRef CAS.
- P. Hashemi, B. Delfan, A. R. Ghiasvand, M. Alborzi and F. Raeisi, A study of the effects of cultivation variety, collection time, and climate on the amount of oleuropein in olive leaves, Acta Chromatogr., 2010, 22, 133–140 CrossRef CAS.
- N. S. A. Malik and J. M. Bradford, Recovery and stability of oleuropein and other phenolic compounds during extraction and processing of olive (Olea europaea L.) leaves, J. Food Agric. Environ., 2008, 6, 8–13 CAS.
- S. Gómez-González, J. Ruiz-Jiménez, F. Priego-Capote and M. D. Luque de Castro, Qualitative and quantitative sugar profiling in olive fruits, leaves, and stems by gas chromatography-tandemmass spectrometry (GC-MS/MS) after ultrasound-assisted leaching, J. Agric. Food Chem., 2010, 58, 12292–12299 CrossRef.
- M. Brenes, A. García, B. de los Santos, E. Medina, C. Romero, A. de Castro and F. Romero, Olive glutaraldehyde-like compounds against plant pathogenic bacteria and fungi, Food Chem., 2011, 125, 1262–1266 CrossRef CAS.
- E. Medina, M. Brenes, A. García, C. Romero and A. de Castro, Bactericidal activity of glutaraldehyde-like compounds from olive products, J. Food Prot., 2009, 72, 2611–2614 CrossRef CAS.
- E. Medina, C. Romero, A. de Castro, M. Brenes and A. García, Inhibitors of lactic acid fermentation in Spanish-style green olive brines of the Manzanilla variety, Food Chem., 2008, 110, 932–937 CrossRef CAS PubMed.
- K. L. Moore, J. R. Patel, D. Jaroni, M. Friedman and S. Ravishankar, Antimicrobial activity of apple, hibiscus, olive, and hydrogen peroxide formulations against Salmonella enterica on organic leafy greens, J. Food Prot., 2011, 74, 1676–1683 CrossRef.
- I. Albertos, R. J. Avena-Bustillos, A. B. Martín-Diana, W. X. Du, D. Rico and T. H. McHugh, Antimicrobial olive leaf gelatin films for enhancing the quality of cold-smoked salmon, Food Packag. Shelf Life, 2017, 13, 49–55 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9fo00698b |
| This journal is © The Royal Society of Chemistry 2019 |