Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Physical effects of dietary fibre on simulated luminal flow, studied by in vitro dynamic gastrointestinal digestion and fermentation
Alba
Tamargo
a,
Carolina
Cueva
a,
M. Dolores
Alvarez
b,
Beatriz
Herranz
b,
M. Victoria
Moreno-Arribas
a and
Laura
Laguna
 *c
*c
aInstitute of Food Science Research (CIAL), CSIC-UAM, C/Nicolás Cabrera 9, 28049, Madrid, Spain
bInstitute of Food Science, Technology and Nutrition (ICTAN), CSIC, C/José Antonio Novais, 10, 28040 Madrid, Spain
cInstitute of Agrochemistry and Food Technology (IATA), CSIC, C/Catedrático Agustín Escardino Benlloch, 7, 46980 Paterna, Spain. E-mail: laura.laguna@csic.es
First published on 13th May 2019
Abstract
During the transit through the gastrointestinal tract, fibre undergoes physical changes not usually included in in vitro digestion studies even though they influence nutrient diffusion and might play a role in gut microbiota growth. The aim of this study was to evaluate how physical fibre properties influence the physical properties of gastrointestinal fluids using a gastrointestinal model (stomach, small intestine, ascending colon, transverse colon, and descending colon) (simgi®). Analysis by rheological and particle size characterisation, microbiota composition and short-chain fatty acid (SCFA) determination allows the achievement of this goal. First, the water-holding capacity (WHC), microstructure, and viscosity of eight different fibres plus agar were tested. Based on the results, potato fibre, hydroxypropyl methylcellulose (HPMC), psyllium fibres, and agar (as a control) were selected for addition to a medium growth (GNMF) that was used to feed the stomach/small intestine and colon compartments in the simgi®. During gastrointestinal digestion, GNMF was collected at 5, 30 and 55 minutes of processing at the gastric stage and after the intestinal stage. Then, samples of GNMF with faecal slurry were collected at 0, 24 and 48 h of colonic fermentation. Results showed fibre-dependence on apparent viscosity. Although psyllium was partially broken down in the stomach (decrease in particle size), it was the most viscous at the colonic stage, opposite to the potato fibre, but both led to the highest total SFCA and acetic acid production profile. On a microbiological level, the most relevant increase of bacterial growth was observed in the faecal Lactobacillus species, especially for HPMC and potato fibre, that were not digested until reaching the colon. Besides fibre fermentability, viscosity also influenced microbial growth, and it is necessary to characterise these changes to understand fibre functionality.
1. Introduction
Over the years and worldwide, different studies and health organisations have been recommending, for a healthy adult, the consumption of 20 to 40 g of fibre per day.1–4 Fibre is an important dietary component, exhibiting many proven health benefits, such as decreased blood cholesterol,5 the prevention and management of diabetes mellitus6 and the improved reduction of constipation. Fibre also helps to promote the satiety feeling which can help with weight control.7,8 All these effects are dependent on the type of fibre. In fact, dietary fibre is a broad category of non-digestible food ingredients. In 2009, the Codex Alimentarius disclosed an updated definition of fibre as “carbohydrate polymers with three or more monomeric units, which are neither digested nor absorbed in the human small intestine and belong to the following categories:– Edible carbohydrate polymers naturally occurring in the food as consumed;
– Edible carbohydrate polymers that have been obtained from food raw material by physical, enzymatic, or chemical means and which have a beneficial physiological effect demonstrated by generally accepted scientific evidence;
– Edible synthetic carbohydrate polymers that have a beneficial physiological effect demonstrated by generally accepted scientific evidence.”9
Therefore, today, non-starch polysaccharides, resistant oligosaccharides and other carbohydrates, such as resistant starch and dextrin, are all considered fibres.10–13 Although fibre is a topic thoroughly investigated regarding quantification, and composition, little research exists regarding its physical changes (i.e., viscosity) through the intestine, changes that are associated with satiety control and/or constipation. Therefore, the study of the viscosity changes along the gastrointestinal tract related to the microbiota growth might shed light on fibre functionality.
Although ideal, it is not possible to study the viscosity changes of fibre along the intestine, in vivo, for technical, ethic and economic reasons. There is a real need for the use of in vitro models, which mimic the physiological conditions occurring during human digestion. Simple static in vitro digestion models, proposed as alternatives to in vivo experiments, are basic and limited when recreating the complexity of the digestive tract. In contrast, dynamic models that allow pH regulation, the flow of the food and the injection of digestive enzymes in real time, in the different compartments of the gastrointestinal tract, are more promising at accurately mimicking the digestive process.14 However, because of its enormous complexity and also the high cost of the installation and optimisation process, there are few simulators of this type in the world.14 Simgi® is a computer-controlled gastrointestinal in vitro model designed to simulate the physiological processes taking place during digestion in the stomach and small intestine. It can also reproduce the colonic microbiota responsible for metabolic bioconversions in the large intestine.15,16 In a previous work, when using simgi®, fed with a hard-to-digest substance (agar), the viscosity itself was an important factor to condition the growth of different bacterial groups.17 However, the study of the effect of dietary fibre viscosity on bacterial growth in an in vitro system has never taken place thus far.
Hence, this work intends to understand how fibres influence the physical properties of gastrointestinal fluids (in the gastrointestinal lumen) using a gastrointestinal model (stomach, small intestine, ascending colon, transverse colon and descending colon) by studying the viscosity, particle size, microbiota and short-chain fatty acids (SCFAs) of the digest, taking samples along the different simgi® compartments and at different times.
2. Materials and methods
2.1 Materials
| Non-fermentable | Minimally fermentable | Intermediate fermentable | ||||||
|---|---|---|---|---|---|---|---|---|
| HPMC | MCG | AF | WFL | WFS | OF | PFa | PSF | |
| Fibres abbreviations correspond to: Hydroxypropyl methylcellulose (HPMC), microcrystalline cellulose (MCG), apple fibre (AF), wheat fibres (WF; L for long fibre and S for short fibre), oat fibre (OF), potato fibre (PF) and psyllium fibre (PSF).a Potato fibre also contained 12% resistant starch and 16% starch. | ||||||||
| Medium particle size (μm) | 80 | >250 (60 mesh): max. 0.1%; >75 (200 mesh): max. 35% | <300 (90% of particles) | 50 | 150 | 250–400 (length) | 80–250 | |
| Purity (%) | 85–95 | 87–92 (8–13 of carboxymethylcellulose sodium) | 60 | 97 | 97 | 96 | 62 | — |
| Insoluble portion (%) | — | — | 75 | 94.5 | 94.5 | — | 56 | 30 |
| Soluble portion (%) | — | — | 25 | 2.5 | 2.5 | — | 6 | 70 |
Henceforward, wheat fibres will be referred to as WF, and the sizes will be L for long fibre and S for short fibre. Further abbreviations include apple fibre (AF), potato fibre (PF), oat fibre (OF), psyllium fibre (PSF), hydroxypropyl methylcellulose (HPMC) and microcrystalline cellulose (MCG).
To prepare the GNMF, all compounds of the GNM and their corresponding weight of fibre or agar were dissolved in 300 ml of distilled water and sterilised at 121 °C for 21 min. All GNMF gel temperatures were lowered to 85 °C inside the autoclave, followed by a further reduction to 37 °C overnight at 150 rpm inside an orbital shaker (Minitron, Infors HT, Switzerland).
2.2. Methods
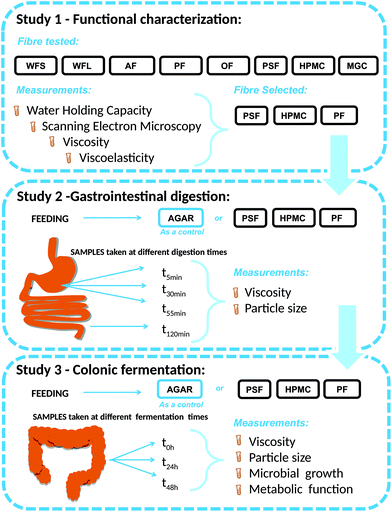
Study 1. Functional characterisation before digestion. Functional characterisation encompassed the evaluation of WHC, microstructure and rheological properties of a range of fibres, followed by the selection of three fibres with different characteristics to feed the stomach/colon.
WHC is the ability to retain water in the internal structure of the fibre. The determination was adapted from Yamazaki et al. (2005).20 0.5 g of fibre was transferred to a 50 mL centrifuge tube. Then, 30 mL of distilled water and GNM at two different pH (pH = 2.3 and pH = 6.3) were added to the tube. All tubes were incubated at 37 °C and stirred at 150 rpm overnight. After, the tubes were centrifuged at 2000g for 10 min in a Rotina 380R (HETTICH, Germany). Each tube was decanted for 10 min and the liquid was separated before it was weighed. The amount of liquid held was calculated by subtracting the weight before liquid treatment and was expressed relative to the dry weight. The WHC of all fibres were determined in triplicate for each liquid (distilled water, GNM pH = 2.3 and pH = 6.3).
Study 2. Gastrointestinal digestions. Simgi® is a computer-controlled dynamic SIMulator of the gastrointestinal tract comprising five successive reactors which are used separately.15,21 In the present work, simgi® modular design was used to simulate digestion in two steps: gastrointestinal digestion (study 2) and colonic fermentation (study 3).
For gastrointestinal digestions, the link between the stomach and small intestine reactors simulated the upper digestion tract. The authors selected the parameters of process simulation based on literature data to mimic in vivo conditions (Bellmann, Lelieveld, Gorissen, Minekus, & Havenaar, 2016) and preliminary experiments.15,22,24
At the gastric level, the peristalsis frequency was set to 10 s−1. The feeding volume of 80 mL of each GNMF was used in addition to a pH curve descending from the initial feeding pH to pH = 2 (fasting conditions) during the gastric digestion stage. Gastric juice (15 mL) was prepared as a solution of pepsin, from porcine gastric mucosa (2000 U mL−1) (Sigma-Aldrich, MERK, USA) and high-purity NaCl (0.9 g L−1) (VWR Chemicals, Avantor®, USA); the solution was kept at 4 °C to avoid autolysis and before it was delivered at 3.9 mL min−1 flow to the gastric compartment. Enzymatic activity (2500 U mg−1) using a haemoglobin substrate was obtained. To control the gastric emptying, the Elashoff power exponential function was used:
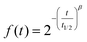 | (1) |
In the small intestinal reactor, the gastric emptying content is mixed gradually (5 mL min−1) with 40 mL of pancreatic juice (of a solution of 12 g L−1 NaHCO3 (VWR Chemicals, Avantor®, USA), 6 g L−1 Oxgall dehydrate fresh bile (Difco™, BD, USA) and 0.9 g L−1 pancreatin from the porcine pancreas (Sigma-Aldrich, MERK, USA)) prepared as21,25 during intestinal digestion (2 h, 37 °C) in anaerobic conditions.
Each of the four different GNMF, with agar, PF, HPMC and PSF, was added separately, in a single dose, and underwent dynamic digestion in the simgi® stomach and small intestine reactors. For viscosity and particle size analysis, samples after 5, 30 and 55 min (T1, T2, and T3) in the gastric stage and at the end of the intestinal stage (120 min) were taken.
Study 3. Colonic fermentation. For colonic fermentations, four simgi® reactors were independently used to control the in vitro conditions (at same pH, temperature and shaking rate).17
GNMF (with agar, PF, HPMC and PSF) was transferred to the four intestinal simgi® compartments with the addition of 20 ml of faecal slurry. The reactors were filled and pre-conditioned with nutritive medium in 300 mL of nutrient medium. Experimental conditions were kept the same for the four compartments during the whole experimental process. Using pH controllers (Unitronic Vision 120™, UNITRONICS®, Israel) pH automatic regulation was kept to 6.3 ± 0.2. The compartments were maintained at 37 °C under anaerobic conditions by continuously flushing nitrogen. For SCFA determination, microbiological and rheological analyses, the samples were taken simultaneously at 0, 24, and 48 h.
The initial characterisation of samples from Study 1 was measured before digestion, at 25 °C. Samples from study 2 (gastrointestinal digestion) and 3 (colonic fermentation) were measured at 37 °C, a value representative of the body temperature. The use of a temperature cover kept the samples at the specified temperatures, which were controlled to be within 0.1 °C by Peltier elements in the lower plate.
To achieve homogenised mechanical equilibrium before measurement, a pre-shearing test on all the samples over 1 min at 100 s−1 at the corresponding temperatures of 25 °C and 37 °C was carried out. The resulting flow curves were obtained as a function of shear rate ranging between 100 to 0.01 s−1. All samples were measured at least in triplicate.
The viscosities in two shear rates between the physiological range (1 and 10 s−1) were chosen26 to help characterise the flow curves of the different samples.
The calibration curves of the analysed compounds were obtained by diluting the original stock standard solution (20 g L−1 of acetic acid, 20 g L−1 of propionic acid and 5 g L−1 of butanoic acid in GNM). The analyses were performed in duplicate.
Principal component analysis (PCA) was performed to study the relationship between physical properties, microbiological growth, and SCFA production. In addition, Pearson's correlation was done to specifically study viscosity and microbial growth. A PCA and Pearson's correlation were obtained using the statistical software package XLSTAT-Sensory (Addinsoft-Barcelona, Spain, version 2018.2).
3. Results and discussion
3.1. Fibre morphology
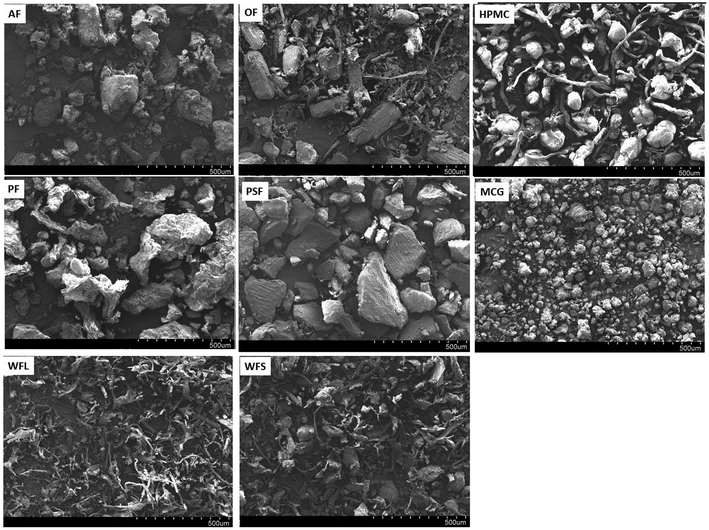
Fibres can bind water in several ways.28 Besides its chemical composition and processing history, fibre hydration properties depend on their physical structures (surface area and particle size).29 Therefore, to understand the behaviour of fibre in the gastrointestinal system in-depth, Fig. 2 shows SEM photographs for the fibres (AF, OF, HPMC, PF, PSF, MCG, WFS and WFL).The apple fibre (AF) shows a rounded structure, with different particle sizes, very heterogeneous distribution and numerous irregularities on its surface. The oat fibre (OF), shows an elongated structure with very heterogeneous fibres of different thicknesses. With the HPMC, two structures can be seen, long fibres and round particles of small size. The potato fibre (PF), presents many irregularities in the size and surfaces of its particles, being clearer at higher magnifications. Psyllium fibre (PSF) shows a regular and compact structure. The MCG fibre corresponds to crystalline methylcellulose, where the particle size is lower than all the previous fibres, and a uniform size is seen in its particles. Regarding the wheat fibres, WFS is a short wheat fibre made up of numerous small fibrils packed together to form a matrix, whilst for WFL, the long wheat fibre, its appearance is similar to that of WFS but presents greater fibril size, with greater particle length and thickness.
3.2. Initial physical characterisation of GNMFs
Gastrointestinal sections are anatomically different from each other, creating different environments and secretions, with one major difference being the pH and another being the gastrointestinal motility of each section. Therefore, the WHC and apparent viscosity of the GNMF (at 3%) were measured before transferring it to simgi® at two different pH (2.3 and 6.3).| Fibre | Water-holding (%) | η a,1 (Pa s) | η a,1 (Pa s) | η a,10 (Pa s) | η a,10 (Pa s) | ||
|---|---|---|---|---|---|---|---|
| Solvent H2O | Solvent GNM at pH = 2.3 | Solvent GNM at pH = 6.3 | pH = 2.3 | pH = 6.3 | pH = 2.3 | pH = 6.3 | |
| A–GEffect of dietary fibre. For each fibre physical property, mean values in the same column without the same letter are significantly different (p < 0.05). a,bEffect of pH. For the same physical property and dietary fibre type, mean values in the same row without the same letter are significantly different (p < 0.05). WFS: wheat fibre short; WFL: wheat fibre long; AF: apple fibre; PF: potato fibre; OF: oat fibre; PSF: psyllium fibre, HPMC: hydroxypropyl methylcellulose; MCG: microcrystalline cellulose. | |||||||
| WFL | 689 ± 52.9Da | 626 ± 66.8Ca | 781 ± 98.3Da | 5.43 ± 0.253Cb | 6.64 ± 0.706B,Ca | 0.983 ± 0.065Cb | 1.26 ± 0.096Ba |
| WFS | 353 ± 5.27Eb | 370 ± 6.84Db | 392 ± 10.7F,Ga | 0.026 ± 0.003Db | 0.314 ± 0.014C,Da | 0.015 ± 0.001Db | 0.103 ± 0.010Ba |
| AF | 521 ± 11.1D,Ea | 605 ± 20.5Ca | 519 ± 62.7E,Fa | 0.174 ± 0.024Db | 0.661 ± 0.015C,Da | 0.059 ± 0.004C,Db | 0.152 ± 0.004Ba |
| PF | 665 ± 20.4Da | 613 ± 62.2Ca | 699 ± 25.9D,Ea | 0.370 ± 0.088Da | 0.181 ± 0.014Db | 0.116 ± 0.016C,Da | 0.051 ± 0.003Bb |
| OF | 269 ± 33.1Ea | 244 ± 6.84Da | 259 ± 10.2Ga | — | — | — | — |
| PSF | 5000 ± 120.6Aa | 2337 ± 86.75Ac | 3450 ± 108.2Ab | 10.2 ± 2.23Bb | 22.2 ± 5.69Aa | 2.82 ± 0.497Bb | 6.23 ± 1.75Aa |
| HPMC | 1784 ± 96.55Ba | 1709 ± 155.7Ba | 1950 ± 123.2Ba | 19.1 ± 2.01Aa | 12.1 ± 2.83Bb | 10.1 ± 0.793Aa | 7.14 ± 1.31Ab |
| MCG | 1404 ± 219.0Ca | 367 ± 18.4Dc | 1083 ± 29.67Cb | 0.285 ± 0.075Db | 0.459 ± 0.054C,Da | 0.051 ± 0.013C,Da | 0.073 ± 0.006Ba |
| Agar | 1274 ± 149.7Ca | 792 ± 41.27Cb | 1075 ± 102.1Ca | 0.206 ± 0.088Db | 4.13 ± 0.235C,Da | 0.038 ± 0.010Db | 1.59 ± 0.056Ba |
In all the mediums and at different pH, psyllium fibre (PSF) had the highest significant (p < 0.05) WHC, followed by hydroxypropyl methylcellulose (HPMC) and microcrystalline cellulose (MCG), which had WHC similar to agar. AF, PF and WFL had similar WHC, while OF, under the three studied conditions, had the lowest WHC.
By using water as a solvent, PSF and MCG also resulted in significantly higher water retention capacities compared with the use of GNM as a solvent at pH 2.3 and 6.3. Attributing this to other polysaccharides in the GNM results in a lack of water availability for these fibres with a high ability for water absorption. pH also had an influence on the WHC, increasing at pH = 6.3 for PSF, MCG and agar.
Except for the long wheat fibre (WFL) and the microcrystalline cellulose (MCG), the remaining samples with higher WHC had more viscosity, as viscosity depends on the degree of hydration.32 In the case of WFL, which had a lower WHC than MCG, the viscosity was significantly higher than the other fibres (MCG, WFS, PF, AF, OF and agar). Although both wheat fibres (WFL and WFS) had the same composition, WFL had a larger particle size (observed in Fig. 2), which was proven to increase the viscosity coefficient.33 This increase resulted from more holes and pores produced, with the ability to hold water, when polysaccharide single chains interact with other chains to form junction zones, trapping larger amounts of water, strongly bound and fixed.28,34
GNMF with psyllium (PSF) has a viscosity, approximately ten times higher than the rest of fibres. Previous studies showed that PSF is a highly branched arabinoxylan polysaccharide consisting of a xylose backbone and arabinose and xylose containing side chains,28 with a negative charge due to ionized carboxyl groups.35 The intermolecular electrostatic repulsions due to equal charges make the chain molecular structures completely extended, in which the adjacent chains form intermolecular cross-links that form a gel.19,35
Regarding the viscosity of the cellulose studied (HPMC and MCG), it can be seen that HPMC viscosity, after the psyllium fibre (PSF), is the greatest and is greater than MCG. Previously, it had been reported that in celluloses, lower viscosities correlated to more degraded cellulose molecules, which consisted of shorter chains,36 as observed in Fig. 2.
The viscosity of agar at pH = 6.3 was higher, as the backbone structure of agar, the agarose, is essentially sulphate-free and consists of alternating chains of β-1,3-D-galactose and α-1,4-3,6-anhydro-L-galactose37 that form a stronger gel at high pH than at low pH.
The viscosity for GNMF with WFL, WFS, AF, PSF, MCG and agar, increased with increasing pH, but for HPMC and PF, the viscosity decreased when increasing pH. Therefore, the influence of pH on viscosity depended on dietary fibre type because there was an optimum pH for each dietary fibre. Today, there is still controversy about the influence of pH on viscosity. It has been stated that the optimum pH of the samples is dependent on the chemical composition of the fibre source,38 so each fibre has an optimum pH. However, one study using the same fibres have claimed that with pH increment, the viscosity increased,39 while other study showed that a decrease in pH produced an increase in viscosity.38 The discrepancy could be related to the non-starch polysaccharide fractions of fibre sources and their purity, as they may be released and broken down upon acidification.40 In the case of HPMC and PF it is apparent that both suffer alkaline depolarisation with a consequent loss of gel strength and viscosity. Furthermore, previous authors have found that acid mediums increase the hydration of HPMC and might be the reason for its high viscosity at low pH in comparison with at high pH.41
At the physiological level, it might be expected that HPMC and PF fibres are more viscous in the stomach (acidic pH), while WFL, WFS, AF, PSF, MCG and agar might be more viscous in the small intestine (neutral pH). However, the rheology of fibres before digestion is not necessarily a reflection of their rheological effects in the gut when ingested, as there is a dilution effect (gastrointestinal tract fluids), different shears, different pH, as well as diffusion throughout the lumen.42,43
It is noteworthy that the medium (GNM) might be influencing viscosity. From one side, the monovalent ions added to adjust the pH of the GNM affects fibres viscosity at both pHs. H+, Na+, and K+ have been reported to interact with hydrocolloids through specific and non-specific interactions, H+ at low pH often because the protonation of carboxylic groups induces weaker gel formations in polysaccharides carrying carboxylic groups.44 From the other side, mucin (4 g L−1) in the GNM may slightly increase the viscosity of all the samples at pH 2.3, as it has the property of increasing its gelation ability by lowering the pH, resulting in the change in viscosity.45 However, because all samples have the same concentration of mucin, it would have affected all the GNMF samples similarly, and as a result, the difference observed is because different fibres or agar were added. A previous study showed that the apparent viscosity of GNM without agar, measured at pH 6.3 and at 1 s−1 shear rate, was 0.005 ± 0.001 Pa s.17 However, the addition of fibres and agar used in this study at 3% (w/w) concentration conferred to the GNMF viscosity range from 0.31 to 22 Pa s (Table 2).
GNMF using OF fibre had no measurable viscosity because of its high instability and precipitation in an aqueous medium.
3.3. Rheological properties of the gastric and small intestinal digestion of GNMF
Table 3 shows viscosity values at 1 and 10 s−1 (ηa1 and ηa10) for the PF, HPMC, PSF and agar GNMF in the stomach at three different times (5, 30 and 55 minutes) through gastric and intestinal processes (after 120 minutes). In all cases, a pronounced diluting effect was observed between the initial feeding (previous section), the gastric compartment and the intestinal compartment. This diluting effect can be attributed to the simulated gastric secretions in the stomach and to the simulated intestinal secretions (pancreatic juice) in the small intestine.| Fibre | Simgi® compartment | η a,1 (Pa s) | η a,10 (Pa s) |
|---|---|---|---|
| A–CEffect of fibre type at each simgi® compartment and time point. For each viscosity, mean values in the same column without the same letter are significantly different (p < 0.05). PF: potato fibre; PSF: psyllium fibre, HPMC: hydroxypropyl methylcellulose. | |||
| Agar | Gastric at 5 min | 0.166 ± 0.027B | 0.030 ± 0.005C |
| PF | 0.020 ± 0.013B | 0.006 ± 0.004C | |
| HPMC | 0.204 ± 0.037B | 0.135 ± 0.004B | |
| PSF | 1.63 ± 0.377A | 0.246 ± 0.045A | |
| Agar | Gastric at 30 min | 0.210 ± 0.006B | 0.040 ± 0.001B |
| PF | 0.063 ± 0.005B | 0.028 ± 0.006B | |
| HPMC | 0.112 ± 0.009B | 0.076 ± 0.007B | |
| PSF | 1.84 ± 0.434A | 0.320 ± 0.110A | |
| Agar | Gastric at 55 min | 0.209 ± 0.019B | 0.042 ± 0.005B |
| PF | 0.029 ± 0.003B | 0.008 ± 0.002B | |
| HPMC | 0.073 ± 0.007B | 0.046 ± 0.002B | |
| PSF | 1.72 ± 0.220A | 0.286 ± 0.048A | |
| Agar | Intestinal | 0.010 ± 0.002B | 0.003 ± 0.000B |
| PF | 0.005 ± 0.003B | 0.001 ± 0.001B | |
| HPMC | 0.010 ± 0.000B | 0.004 ± 0.000B | |
| PSF | 0.376 ± 0.035A | 0.062 ± 0.006A | |
In line with the rheological behaviour of the feeding products, along the gastrointestinal tract, all samples exhibited similar flow behaviour at all concentrations and digestion stages, showing a non-Newtonian behaviour or a pseudoplastic behaviour.
In the gastric compartment, in comparison with the other fibres (PF and HPMC) and the control (agar), psyllium fibre (PSF) showed the highest viscosity, with no statistical variance with time. Although not statistically significant, HPMC showed a viscosity decrease over time in the stomach. Previous authors have stated that HPMC is stable over the pH range of 3.0–11.0 and is enzyme resistant.46 In this work, as the pH was below 3.0 (pHstomach = 2.3), it is believed that this was the cause of the viscosity decrease.
Furthermore, regardless of the simgi® compartment, GNMF with PSF had the highest viscosity, while those with added potato fibre (PF) had the lowest viscosity (Table 2), although with no difference from HPMC or the control with agar.
The lowest viscosity values corresponding to GNMF containing PF could be because potato fibre is more insoluble and had less WHC (Table 2) than HPMC and PSF. However, though it provides less viscosity to the viscosity of the luminal fluid, this does not mean that it does not play a role in intestinal health, as it can be fermented (to be discussed in the next section) or, if it is not digested and highly insoluble, might promote a laxative effect. Laxative effects can occur through two different mechanisms. One is by mechanically irritating the large bowel mucosa with the presence of insoluble particles.47 The other is by the production of a gel-forming fibre (psyllium) that will resist dehydration (by water reabsorption) in the large bowel.47
Psyllium fibre (PSF) was very viscous during the gastric and small intestinal digestive processes. These results are in accordance with the previously reported psyllium properties, lowering after-meal glucose and insulin levels, due to a delay in the gastric emptying, and also lowering cholesterol by impeding enzyme–substrate interaction. This second effect is highly dependent on the viscosity.48,49
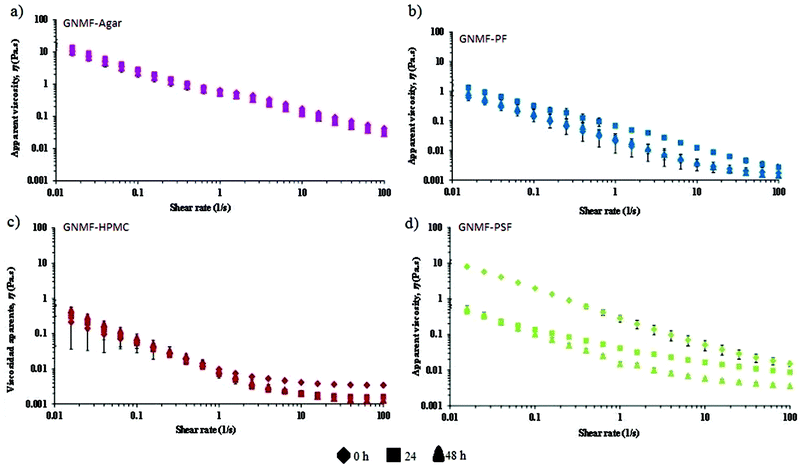
3.4. Rheological properties during the fermentation of inoculated GNM gels with added fibres
The flow curves of the GNMFs with added faecal slurries at different times (0, 24 and 48 h) are shown in Fig. 3.Agar gels (control) were significantly (p < 0.05) the most viscous along with fermentation in the colonic vessels, confirming that human intestinal microbiota does not degrade bacteriological agar.
Among all the fibres presented in Fig. 3, psyllium fibre (PSF) experienced a major drop in viscosity over time (Fig. 3d). For potato fibre (PF) (Fig. 3b), the viscosities were slightly higher after 24 and 48 h than at 0 h, likely caused by the resistant starch content of this fibre (Table 1).
At 0 h, it can be noted that viscosity values for the PSF gel were still significantly higher than those of the PF and HPMC gels, which were the lowest without significant differences between them. In contrast, at the end of the fermentation period (48 h), the PSF gel exhibited much lower viscosities than at previous times, reflecting that intestinal microbiota highly fermented this GNMF.
As mentioned in the previous sections, the secretion of fluids was mimicked according to the gastric secretions that caused the sequential loss in viscosity along the gastrointestinal tract. However, under normal conditions, most of the fluid in the small intestine is absorbed. A drawback to in vitro studies is that water and nutrient absorption, which plays a role in the physicochemical properties, cannot be taken into account.50 If it was possible to add water absorption to an in vitro system, an increase in the viscosity of the chyme would be expected, especially for the gel-forming fibre,19 psyllium.
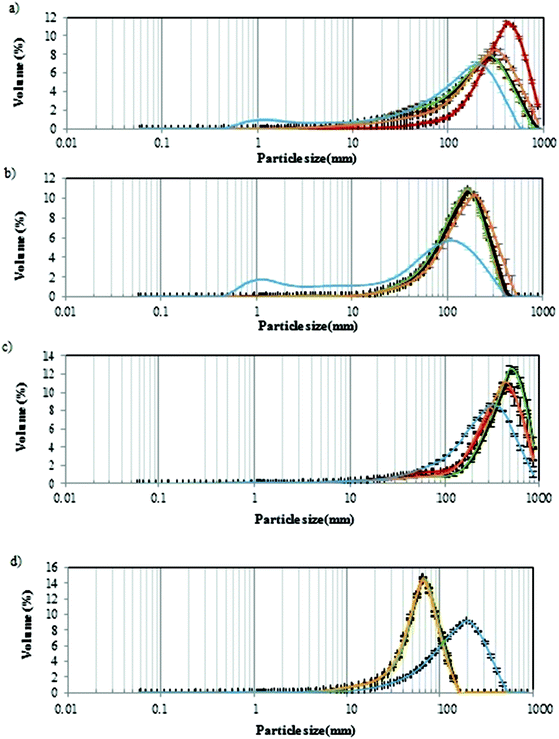
3.5. Particle size measurement
The particle size of the digest depends on the medium in which the fibre is present (pH, composition and enzymatic presence), as well as the mechanical forces created by simulated peristaltic movements.51 Particle size can reflect fibre fragmentation and breakdown through gastrointestinal digestion and fibre agglomerate formation. The results represent the maximum diameter or a spherical particle. However, the real shape of GNMF particles is unknown, and the sizes provided were based in a calculation model.In Fig. 4, the particle size distribution of the GNM gels with added PSF (a), HPMC (b) and PF (c) fibres and agar gels (d) during the gastrointestinal digestion at each simgi® compartment is shown. All samples show a mono-modal distribution during the three different times (T1, T2 and T3) of gastric and intestinal digestions. Agar gel (Fig. 4d) showed a larger and narrower peak with only one population of particles ranging between 10 and 100 μm. While for the GMNF with added fibres, the peak was greater and shifted to the right with the particle population between 10 and 1000 μm. These observations meant that agar, the smaller hydrocolloid, was not digested at the gastric, intestinal or colon level maintaining small particles sizes at both levels. However, GNMF with fibres (PF, HPMC and PSF) had a significant size drop in the colon, in correspondence with microbial fermentation. Meaning that, as expected, the volume of big particles decreases as digestion progresses.51
Previous sources show that hydrophilic matrices reduce in size and dissolve after initially swelling, as they pass through the gastrointestinal tract.52 This could explain the slight increase in HPMC particles.
3.6. Microbial community on a colonic level with different GNMF
In order to stimulate the proliferation of the intestinal microbiota, fibres have to remain relatively intact through the large intestine.53Preliminary experiments confirmed that human intestinal microbiota did not degrade agar with significant effect.17 In addition, it was shown that agar could increase the intestinal viscosity at concentrations higher than 0.30%. Therefore, agar itself does not influence the growth of colonic microbiota because of fermentation and can be used as a control to compare fibre behaviour in simgi® tests.
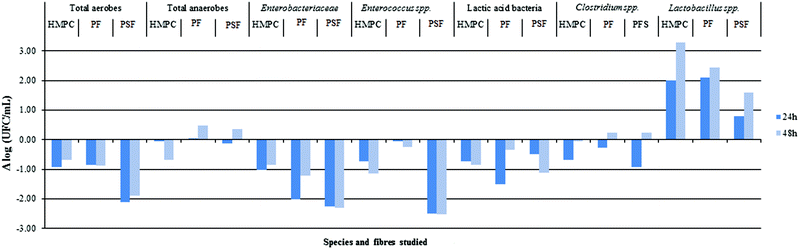
Fig. 5 shows the differences between the different GNMF, with the increase to agar microbial growth [Δlog (CFU ml−1)] as a function of fibre type, different bacterial groups and from the time 0 to 24 and 48 h of the fermentation process.
As expected, at time 0 h, no significant differences were observed for any of the groups tested regarding agar (results not shown). At time 24 and 48 h, there were significant differences among all bacterial groups. However, from a microbiological point of view, statistically significant values are greater than Δlog ≥ 1.54
The most relevant increase of bacterial growth was observed for HPMC, potato fibre (PF) (24 and 48 h) and psyllium fibre (PSF) (48 h) on the Lactobacillus species (LAMVAB media). LAMVAB media is highly selective for lactobacilli, particularly human faecal species like Lactobacillus paracasei rhamnosus, Lactobacillus acidophilus, Lactobacillus plantarum and Lactobacillus reuteri. These species are described as possible probiotic species55 and have been isolated on this media.56 The lactobacilli isolation meant that the studied fibres could help the growth of this species in colonic conditions. However, further studies are needed to establish this relationship.
After 24 h of colonic fermentation, the PSF fibre showed a significant reduction in the growth of total aerobes, Enterobacteriaceae and Enterococcus spp. groups. The PF for Enterobacteriaceae and lactic acid bacteria groups showed the same trend. Subsequently, after 48 h of incubation, there was an observation that PSF fibre led to a significant reduction in the growth of total aerobes, Enterobacteriaceae, Enterococcus spp. and lactic bacteria groups. Similarly, there was a decrease in the growth of Enterobacteriaceae for PF and Enterococcus spp. for HPMC. This is in accordance with previous results,17 where the total aerobes and Enterococcus spp. showed lower growth than the rest of the bacterial groups studied. For the remaining studied fibres and bacterial groups, the current authors found no significant microbiological differences (considered as statistically significant being greater than one logarithm) regarding agar.
3.7. SCFA production during colonic fermentation after feeding simgi® with different GNMF
Fig. 6 shows the production of SCFAs after 24 and 48 h with different fibres. As stated previously, agar has low fermentation rates, although some SCFA production exists. These SCFAs originate in GNM that contained arabinogalactan, pectin, xylan and other fermentable sources. Therefore, the differences between SCFA production and the colonic fermentation of GNMF are given.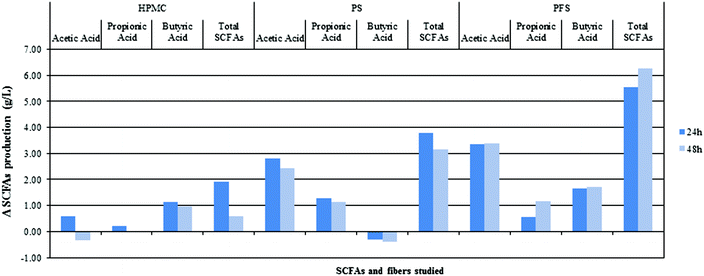 | ||
| Fig. 6 Difference in SCFA production between agar and HPMC (hydroxypropylmethylcellulose), PF (potato fibres) and PSF (psyllium fibre) for acetic acid, propionic acid, butyric acid and total SCFAs. | ||
The quantity and type of SCFA produced were dependent on the type of fibre and time, such that a great increase was observed after 24 h of colonic fermentation. Acetic acid production was as expected, especially for PSF and PF, with a greater SCFA concentration. However, HPMC acetic acid concentration did not show significant differences from agar. All SCFA production followed a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 proportion between acetic and propionic acid. Propionic acid production was significantly higher for PF followed by PSF, while the concentration of butyric acid was significantly higher for PSF and HPMC gels in comparison with agar.
1 proportion between acetic and propionic acid. Propionic acid production was significantly higher for PF followed by PSF, while the concentration of butyric acid was significantly higher for PSF and HPMC gels in comparison with agar.
The results of this study showed that all fibres significantly favoured total SCFA production, although the production profiles differ for HPMC, PF and PSF. This could be explained by their different origin sources, signifying different chemical compositions and different nutritional sources for gut microbiota.57
Despite the chemical composition differences, physical properties, such as the structural features of each fibre and the viscosity of the studied GNMF, could also influence the differences observed in the SCFA production profile. According to other authors,18 different gels with different viscosities of the same nutrition medium, i.e., with the same nutritional source for gut microbiota, resulted in significantly different bacterial growth during colonic fermentation. These, and not only fibre nutritional composition, can impact fibre utilisation by the gut microbiota. Although fibre chemical composition is a key factor, other factors previously proposed, such as physical form, can impact the microbiota and its fermentation.10
The production of SCFA is considered as beneficial to the host because they prevent and protect humans against different diseases and have an important role in maintaining human health inflammatory homeostasis.58,59 Therefore, it could be a key factor in understanding how physical properties, such as viscosity, are related to SCFA production, for which more studies will be necessary.
3.8. Relation among measured physical properties and microbiota growth
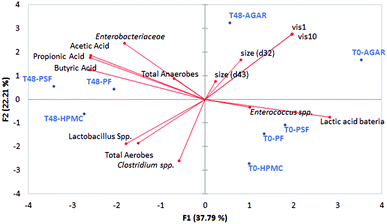
As a summary of the colonic results in Fig. 7, a principal component analysis was shown with the data from particle size, viscosity, microbiota growth, and short chain fatty acids production.It can be observed that the most viscous sample at colonic level was the control (agar sample), also having a larger particle size; these two facts are related to the non-digestibility of the agar. The other three fibres used (psyllium, potato and HPMC) were fermented, having a lower viscosity impact in the colon GNMF samples and also had smaller particles.
Different physical characteristics of the fibres can be linked to different beneficial health effects,19 marking the importance of the physical measurements of fibres in vitro. As it was presented in Table 1, fibres have different solubilities, meaning that the number of soluble solids is different. However, solubility does not allow the prediction of viscosity. Among fibres, there are also soluble fibres such as inulin or fructo-oligosaccharides, which are highly beneficial but do not provide viscosity to intestinal lumen.60 In this case, if there a lower digestibility of the samples (agar), there is more of an influence on the physical properties of the gastrointestinal lumen, through increasing the viscosity and the presence of larger particles, leading to laxation effects or improvement in glucose and insulin metabolism, according to the bibliography.8
A correlation test of viscosity and microbial growth was performed and is presented in Table 4. It can be observed that total anaerobes were related to the highest viscosities, but this was not linked with any specific anaerobic group.
| Variables | Total aerobes | Enterobacteriaceae | Total anaerobes | Enterococcus spp. | Lactic acid bacteria | Clostridium spp. | Lactobacillus spp. | Viscosity at 1 s−1 | Viscosity at 10 s−1 | Size (d32) | Size (d43) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Values in bold are different from 0 with a significance level alpha = 0.05. | |||||||||||
| Total Aerobes | 1 | −0.714 | −0.129 | −0.462 | −0.743 | 0.735 | 0.901 | −0.989 | −0.993 | −0.342 | 0.004 |
| Enterobacteriaceae | −0.714 | 1 | −0.332 | 0.866 | 0.660 | −0.621 | −0.356 | 0.804 | 0.791 | 0.358 | −0.110 |
| Total Anaerobes | −0.129 | −0.332 | 1 | −0.052 | 0.489 | 0.510 | −0.474 | 0.080 | 0.074 | 0.673 | 0.868 |
| Enterococcus spp. | −0.462 | 0.866 | −0.052 | 1 | 0.773 | −0.147 | −0.165 | 0.586 | 0.558 | 0.697 | 0.325 |
| Lactic acid bacteria | −0.743 | 0.660 | 0.489 | 0.773 | 1 | −0.144 | −0.683 | 0.792 | 0.773 | 0.880 | 0.605 |
| Clostridium spp. | 0.735 | −0.621 | 0.510 | −0.147 | −0.144 | 1 | 0.513 | −0.714 | −0.731 | 0.340 | 0.680 |
| Lactobacillus spp. | 0.901 | −0.356 | −0.474 | −0.165 | −0.683 | 0.513 | 1 | −0.840 | −0.850 | −0.371 | −0.197 |
| Viscosity at 1 s−1 | −0.989 | 0.804 | 0.080 | 0.586 | 0.792 | −0.714 | −0.840 | 1 | 0.999 | 0.406 | 0.022 |
| Viscosity at 1 0s−1 | −0.993 | 0.791 | 0.074 | 0.558 | 0.773 | −0.731 | −0.850 | 0.999 | 1 | 0.379 | 0.000 |
| Size (d32) | −0.342 | 0.358 | 0.673 | 0.697 | 0.880 | 0.340 | −0.371 | 0.406 | 0.379 | 1 | 0.889 |
| Size (d43) | 0.004 | −0.110 | 0.868 | 0.325 | 0.605 | 0.680 | −0.197 | 0.022 | 0.000 | 0.889 | 1 |
4. Conclusion
This work presents new knowledge about how the physical properties of fibres influence the outcomes in a gastrointestinal model. Evidence shows that fibres with higher water-holding capacity provide higher viscosity but depend on the pH at each gastrointestinal section.Initial viscosity and WHC do not have a direct correlation with SCFA production and bacterial growth. SCFA production was higher for potato fibre (PF), with a low viscosity, but also for psyllium fibre (PSF), which had a high viscosity. However, the physical measurements along the intestinal tract allow us to have a better understanding of the fibre effect. As shown in this study, gastrointestinal conditions (pH, temperature, and enzymes) influence the effect of fibres on microbiota growth and can be potentially correlated to human physiology effects (satiety, postprandial glycaemia and nutrition diffusion across the mucosal membrane, among others).
This study highlights the importance of including rheological studies for a deeper understanding of the human microbiota, a crucial process for the design and screening of functional foods.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors of this work were funded by the Spanish MINECO project (AGL2015-64522-C2-R-01). L. L. would like to thank the Spanish “Juan de la Cierva” program for her contract (ref FJCI-2014-19907 and IJCI-2016-27427). C. C. would like to thank the Comunidad de Madrid Program (ALIBIRD2020-CM P2018/BAA-4343) for her postdoctoral research contract.References
- J. L. Slavin, Position of the American Dietetic Association: health implications of dietary fiber, J. Am. Diet. Assoc., 2008, 108, 1716–1731 CrossRef PubMed.
- J. Salas-Salvadó, M. A. Rubio, M. Barbany, B. Moreno and G. C. de la SEEDO, Consenso SEEDO 2007 para la evaluación del sobrepeso y la obesidad y el establecimiento de criterios de intervención terapéutica, Med. Clin., 2007, 128, 184–196 CrossRef.
- S. A. Bingham, N. E. Day, R. Luben, P. Ferrari, N. Slimani, T. Norat, F. Clavel-Chapelon, E. Kesse, A. Nieters, H. Boeing, A. Tjϕnneland, K. Overvad, C. Martinez, M. Dorronsoro, C. A. Gonzalez, T. J. Key, A. Trichopoulou, A. Naska, P. Vineis, R. Tumino, V. Krogh, H. B. Bueno-de-Mesquita, P. H. M. Peeters, G. Berglund, G. Hallmans, E. Lund, G. Skeie, R. Kaaks and E. Riboli, Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study, Lancet, 2003, 361, 1496–1501 CrossRef.
- J. W. Anderson, P. Baird, R. H. Davis, S. Ferreri, M. Knudtson, A. Koraym, V. Waters and C. L. Williams, Health benefits of dietary fiber, Nutr. Rev., 2009, 67, 188–205 CrossRef.
- J. A. Marlett and M. J. Longacre, Comparison of in vitro and in vivo measures of resistant starch in selected grain products, Cereal Chem., 1996, 73, 63–68 CAS.
- M. Chandalia, A. Garg, D. Lutjohann, K. von Bergmann, S. M. Grundy and L. J. Brinkley, Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus, N. Engl. J. Med., 2000, 342, 1392–1398 CrossRef CAS PubMed.
- D. J. Jenkins, T. M. Wolever, A. L. Jenkins and R. H. Taylor, in Dietary Fiber, Springer, 1986, pp. 69–80 Search PubMed.
- D. J. A. Jenkins, A. Marchie, L. S. A. Augustin, E. Ros and C. W. C. Kendall, Viscous dietary fibre and metabolic effects, Clin. Nutr. Suppl., 2004, 1, 39–49 CrossRef.
- F. Joint, WHO Food Standards Programme, Secretariat of the CODEX Alimentarius Commission: CODEX Alimentarius (CODEX) Guidelines on Nutrition Labeling CAC/GL 2–1985 as Last Amended 2010, FAO, Rome, 2010 Search PubMed.
- B. R. Hamaker and Y. E. Tuncil, A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota, J. Mol. Biol., 2014, 426, 3838–3850 CrossRef CAS PubMed.
- D. Burkitt and H. Trowell, Dietary fibre and western diseases, Ir. Med. J., 1977, 70, 272 CAS.
- C. M. Guinane and P. D. Cotter, Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ, Ther. Adv. Gastroenterol., 2013, 6, 295–308 CrossRef PubMed.
- R. Guiné, J. Duarte, M. Ferreira, P. Correia, M. Leal, I. Rumbak, I. Barić, D. Komes, Z. Satalić and M. Sarić, Knowledge about sources of dietary fibres and health effects using a validated scale: a cross-country study, Public Health, 2016, 141, 100–112 CrossRef PubMed.
- D. Dupont, M. Alric, S. Blanquet-Diot, G. Bornhorst, C. Cueva, A. Deglaire, S. Denis, M. Ferrua, R. Havenaar and J. Lelieveld, Can dynamic in vitro digestion systems mimic the physiological reality?, Crit. Rev. Food Sci. Nutr., 2018, 1–17 CrossRef CAS PubMed.
- C. Cueva, A. Jiménez-Girón, I. Muñoz-González, A. Esteban-Fernández, I. Gil-Sánchez, M. Dueñas, P. J. Martín-Álvarez, M. A. Pozo-Bayón, B. Bartolomé and M. V. Moreno-Arribas, Application of a new Dynamic Gastrointestinal Simulator (SIMGI) to study the impact of red wine in colonic metabolism, Food Res. Int., 2015, 72, 149–159 CrossRef CAS.
- E. Barroso, C. Cueva, C. Peláez, M. C. Martínez-Cuesta and T. Requena, in The Impact of Food Bioactives on Health: in vitro and ex vivo models, ed. K. Verhoeckx, P. Cotter, I. López-Expósito, C. Kleiveland, T. Lea, A. Mackie, T. Requena, D. Swiatecka and H. Wichers, Springer International Publishing, Cham, 2015, pp. 319–327, DOI:10.1007/978-3-319-16104-4_28.
- A. Tamargo, C. Cueva, M. D. Álvarez, B. Herranz, B. Bartolomé, M. V. Moreno-Arribas and L. Laguna, Influence of viscosity on the growth of human gut microbiota, Food Hydrocolloids, 2018, 77, 163–167 CrossRef CAS.
- J. F. Zaragozano, G. R. Martínez, M. G. B. Lozano, L. A. M. Aznar, B. Tresaco, J. M. G. Otero and M. A. B. Sánchez, Psyllium fibre and the metabolic control of obese and adolescents, J. Physiol. Biochem., 2003, 59, 235–242 CrossRef.
- J. W. McRorie Jr. and N. M. McKeown, Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber, J. Acad. Nutr. Diet., 2017, 117, 251–264 CrossRef.
- E. Yamazaki, K. Murakami and O. Kurita, Easy Preparation of Dietary Fiber with the High Water-Holding Capacity from Food Sources, Plant Foods Hum. Nutr., 2005, 60, 17–23 CrossRef CAS.
- E. Barroso, C. Cueva, C. Peláez, M. C. Martínez-Cuesta and T. Requena, Development of human colonic microbiota in the computer-controlled dynamic SIMulator of the GastroIntestinal tract SIMGI, LWT – Food Sci. Technol., 2015, 61, 283–289 CrossRef CAS.
- B. Miralles, R. d. Barrio, C. Cueva, I. Recio and L. Amigo, Dynamic gastric digestion of a commercial whey protein concentrate, J. Sci. Food Agric., 2018, 98, 1873–1879 CrossRef CAS PubMed.
- J. D. Elashoff, T. J. Reedy and J. H. Meyer, Analysis of gastric emptying data, Gastroenterology, 1982, 83, 1306–1312 CAS.
- S. Bellmann, J. Lelieveld, T. Gorissen, M. Minekus and R. Havenaar, Development of an advanced in vitro model of the stomach and its evaluation versus human gastric physiology, Food Res. Int., 2016, 88, 191–198 CrossRef CAS.
- A. Ferreira-Lazarte, F. J. Moreno, C. Cueva, I. Gil-Sánchez and M. Villamiel, Behaviour of citrus pectin during its gastrointestinal digestion and fermentation in a dynamic simulator (simgi®), Carbohydr. Polym., 2019, 207, 382–390 CrossRef CAS PubMed.
- A. K. Hardacre, R. G. Lentle, S.-Y. Yap and J. A. Monro, Does viscosity or structure govern the rate at which starch granules are digested?, Carbohydr. Polym., 2016, 136, 667–675 CrossRef CAS PubMed.
- B. Tepsongkroh, T. Harnsilawat, P. Maisuthisakul and W. Chantrapornchai, Influence of Polyglycerol Polyricinoleate and Biopolymers on Physical Properties and Encapsulation Efficiency of Water-in-Oil-in-Water Emulsions Containing Mango Seed Kernel Extract, J. Dispersion Sci. Technol., 2015, 36, 1126–1133 CrossRef CAS.
- M. F. Chaplin, Fibre and water binding, Proc. Nutr. Soc., 2003, 62, 223–227 CrossRef CAS PubMed.
- M. C. Ralet-Renard, Pectins: their origin, structure and function, Advanced Dietary Fibre Technology, Blackwell Science, 2001 Search PubMed.
- A. K. Hardacre, S. Y. Yap, R. G. Lentle and J. A. Monro, The effect of fibre and gelatinised starch type on amylolysis and apparent viscosity during in vitro digestion at a physiological shear rate, Carbohydr. Polym., 2015, 123, 80–88 CrossRef CAS PubMed.
- G. R. Sanderson, Polysaccharides in foods, Food Technol., 1981, 35, 50–57 CAS.
- V. Vuksan, S. Panahi, M. Lyon, A. L. Rogovik, A. L. Jenkins and L. A. Leiter, Viscosity of fiber preloads affects food intake in adolescents, Nutr., Metab. Cardiovasc. Dis., 2009, 19, 498–503 CrossRef CAS.
- T. Takahashi and T. Sakata, Large particles increase viscosity and yield stress of pig cecal contents without changing basic viscoelastic properties, J. Nutr., 2002, 132, 1026–1030 CrossRef CAS.
- R. Mongeau and R. Brassard, Insoluble dietary fiber from breakfast cereals and brans: bile salt binding and water-holding capacity in relation to particle size, Cereal Chem., 1982, 59(5), 413–417 Search PubMed.
- A. Farahnaky, H. Askari, M. Majzoobi and G. Mesbahi, The impact of concentration, temperature and pH on dynamic rheology of psyllium gels, J. Food Eng., 2010, 100, 294–301 CrossRef CAS.
- M. V. Scatolino, D. W. Silva, L. Bufalino, G. H. Denzin Tonoli and L. M. Mendes, Influence of cellulose viscosity and residual lignin on water absorption of nanofibril films, Procedia Eng., 2017, 200, 155–161 CrossRef CAS.
- M. Glicksman, Food Hydrocolloids, 1983, vol. 2, p. 2 Search PubMed.
- D. Cameron-Smith, G. Collier and K. O'dea, Effect of soluble dietary fibre on the viscosity of gastrointestinal contents and the acute glycaemic response in the rat, Br. J. Nutr., 1994, 71, 563–571 CrossRef CAS.
- A. Bobboi and A. Stephens, The effects of electrolyte and hydrogen ion concentrations on guar gum and glucose tolerance following intraduodenal administration, Nutr. Res., 1996, 16, 1403–1409 CrossRef CAS.
- C. L. Dikeman, M. R. Murphy and G. C. Fahey, Dietary fibers affect viscosity of solutions and simulated human gastric and small intestinal digesta, J. Nutr., 2006, 136, 913–919 CrossRef CAS.
- K. Mitchell, J. Ford, D. Armstrong, P. Elliott, J. Hogan and C. Rostron, The influence of the particle size of hydroxypropylmethylcellulose K15M on its hydration and performance in matrix tablets, Int. J. Pharm., 1993, 100, 175–179 CrossRef CAS.
- I. Brownlee, The impact of dietary fibre intake on the physiology and health of the stomach and upper gastrointestinal tract, Bioact. Carbohydr. Diet. Fibre, 2014, 4, 155–169 CrossRef CAS.
- H. D. Goff, N. Repin, H. Fabek, D. El Khoury and M. J. Gidley, Dietary fibre for glycaemia control: Towards a mechanistic understanding, Bioact. Carbohydr. Diet. Fibre, 2018, 14, 39–53 CrossRef CAS.
- Z. Gao, Y. Fang, Y. Cao, H. Liao, K. Nishinari and G. O. Phillips, Hydrocolloid-food component interactions, Food Hydrocolloids, 2017, 68, 149–156 CrossRef CAS.
- J. P. Celli, B. S. Turner, N. H. Afdhal, R. H. Ewoldt, G. H. McKinley, R. Bansil, S. Erramilli and S. Erramilli, Rheology of gastric mucin exhibits a pH-dependent sol-gel transition, Biomacromolecules, 2007, 8, 1580–1586 CrossRef CAS PubMed.
- C. L. Li, L. G. Martini, J. L. Ford and M. Roberts, The use of hypromellose in oral drug delivery, J. Pharm. Pharmacol., 2005, 57, 533–546 CrossRef CAS.
- J. McRorie, S. Pepple and C. Rudolph, Effects of fiber laxatives and calcium docusate on regional water content and viscosity of digesta in the large intestine of the pig, Dig. Dis. Sci., 1998, 43, 738–745 CrossRef CAS PubMed.
- A. F. Cicero, G. Derosa, M. Bove, F. Imola, C. Borghi and A. V. Gaddi, Psyllium improves dyslipidaemia, hyperglycaemia and hypertension, while guar gum reduces body weight more rapidly in patients affected by metabolic syndrome following an AHA Step 2 diet, Med. J. Nutrition Metab., 2010, 3, 47–54 CrossRef.
- J. W. Anderson, L. D. Allgood, A. Lawrence, L. A. Altringer, G. R. Jerdack, D. A. Hengehold and J. G. Morel, Cholesterol-lowering effects of psyllium intake adjunctive to diet therapy in men and women with hypercholesterolemia: meta-analysis of 8 controlled trials, Am. J. Clin. Nutr., 2000, 71, 472–479 CrossRef CAS PubMed.
- S. Fiszman and P. Varela, The role of gums in satiety/satiation. A review, Food Hydrocolloids, 2013, 32, 147–154 CrossRef CAS.
- F. Kong and R. P. Singh, A human gastric simulator (HGS) to study food digestion in human stomach, J. Food Sci., 2010, 75, E627–E635 CrossRef CAS.
- S. C. Joshi, Sol-gel behavior of hydroxypropyl methylcellulose (HPMC) in ionic media including drug release, Materials, 2011, 4, 1861–1905 CrossRef CAS PubMed.
- J. W. McRorie Jr., Evidence-based approach to fiber supplements and clinically meaningful health benefits, part 1: What to look for and how to recommend an effective fiber therapy, Nutr. Today, 2015, 50, 82 CrossRef PubMed.
- I. Gil-Sánchez, C. Cueva, M. Sanz-Buenhombre, A. Guadarrama, M. V. Moreno-Arribas and B. Bartolomé, Dynamic gastrointestinal digestion of grape pomace extracts: Bioaccessible phenolic metabolites and impact on human gut microbiota, J. Food Compos. Anal., 2018, 68, 41–52 CrossRef.
- N. P. Shah, Functional cultures and health benefits, Int. Dairy J., 2007, 17, 1262–1277 CrossRef.
- R. Hartemink, V. Domenech and F. Rombouts, LAMVAB—a new selective medium for the isolation of lactobacilli from faeces, J. Microbiol. Methods, 1997, 29, 77–84 CrossRef CAS.
- C. M. Sawicki, K. A. Livingston, M. Obin, S. B. Roberts, M. Chung and N. M. McKeown, Dietary Fiber and the Human Gut Microbiota: Application of Evidence Mapping Methodology, Nutrients, 2017, 9, 125 CrossRef PubMed.
- H. L. Simpson and B. J. Campbell, Review article: dietary fibre–microbiota interactions, Aliment. Pharmacol. Ther., 2015, 42, 158–179 CrossRef CAS PubMed.
- M. L. Dreher, in Dietary Fiber in Health and Disease, ed. M. L. Dreher, Springer International Publishing, Cham, 2018, pp. 41–66, DOI:10.1007/978-3-319-50557-2_3.
- Food and Nutrition Board, I. O. M., Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients), A report of the Panel on Macronutrients, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, 2005 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |