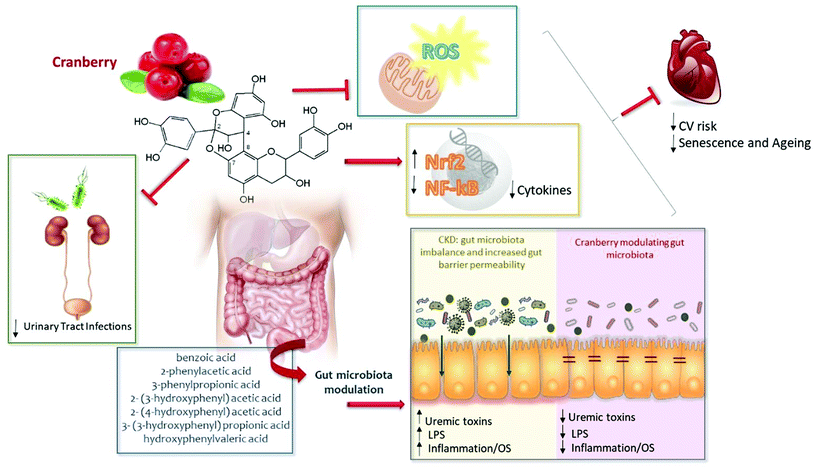
Cranberries – potential benefits in patients with chronic kidney disease
Livia
de Almeida Alvarenga
 a,
Natália Alvarenga
Borges
bc,
Laís de Souza Gouveia
Moreira
b,
Karla Thaís
Resende Teixeira
b,
José Carlos
Carraro-Eduardo
d,
Lu
Dai
e,
Peter
Stenvinkel
e,
Bengt
Lindholm
e and
Denise
Mafra
*abc
a,
Natália Alvarenga
Borges
bc,
Laís de Souza Gouveia
Moreira
b,
Karla Thaís
Resende Teixeira
b,
José Carlos
Carraro-Eduardo
d,
Lu
Dai
e,
Peter
Stenvinkel
e,
Bengt
Lindholm
e and
Denise
Mafra
*abc
aGraduate Program in Medical Sciences, Fluminense Federal University (UFF), Niterói, RJ, Brazil. E-mail: dmafra30@gmail.com
bGraduate Program in Nutrition Sciences, Fluminense Federal University (UFF), Niterói, RJ, Brazil
cGraduate Program in Cardiovascular Sciences, Fluminense Federal University (UFF), Niterói, RJ, Brazil
dMedicine Faculty, Fluminense Federal University (UFF), Niterói, RJ, Brazil
eDivision of Renal Medicine and Baxter Novum, Department of Clinical Science, Technology and Intervention, Karolinska Institutet, Stockholm, Sweden
First published on 29th May 2019
Abstract
Patients with chronic kidney disease (CKD) present many complications that potentially could be linked to increased cardiovascular mortality such as inflammation, oxidative stress, cellular senescence and gut dysbiosis. There is growing evidence suggesting that nutritional strategies may reduce some of these complications. Clinical studies suggest that supplementation of cranberries may have beneficial effects on human health such as prevention of urinary tract infections. More recently, the anti-inflammatory and anti-oxidant effects as well as modulation of gut microbiota provided by cranberry phytochemicals have drawn more attention. A better understanding of possible effects and mechanisms of action of cranberry supplementation in humans could inform researchers about warranted future directions for clinical studies targeting these complications in CKD patients by applying nutritional strategies involving cranberry supplementation.
Introduction
Chronic kidney disease (CKD) is recognized as a global public health problem.1 Advanced stages of CKD are associated with complications such as malnutrition, edema, constipation, increased stress (physical, psychological, or pharmacological), decreased quality of life and reduced life expectancy.2,3 Oxidative stress and inflammation are prevalent in this population, due to an imbalance between reactive oxygen species (ROS) and endogenous antioxidant defense mechanisms.4,5 Additionally, gut microbiota imbalance and increased gut barrier permeability have been reported as sources of oxidative stress and inflammation in CKD. These common findings are considered critical factors for CKD progression and development of cardiovascular disease (CVD), which is the major cause of premature deaths in CKD.6It has been suggested that bioactive compounds with anti-inflammatory and anti-oxidant properties, present in vegetables and fruits, can attenuate the pro-atherogenic effects of the uremic milieu. In this context, berries would be of interest to study further.7–10 American cranberries (Vaccinium macrocarpon Ait.) are rich in bioactive compounds, especially procyanidins, which are potent antioxidants and effective in management of infectious diseases, oxidative stress and inflammation.11–15
For many years, studies have confirmed that some components of cranberry exert beneficial effects in the urinary tract and protect against urinary tract infections.16–19 In addition, it is possible that cranberries may also attenuate oxidative stress and inflammation, and improve the gut microbiota imbalance in CKD patients. In this review we discuss the possible underlying mechanisms of action of cranberries in CKD.
Chronic kidney disease
Systemic inflammation and oxidative stress are established risk factors for increased cardiovascular mortality linked to a premature aging phenotype in CKD.20 While cellular and molecular mechanisms that contribute to the high prevalence of inflammation in CKD are complex, redox-sensitive signaling pathways are involved in persistent uremic inflammation.5 The dysregulated systemic redox balance can be detected at early stages of CKD and increases along the progression of the disease.21,22 In this situation, the oxidative stress condition is not accompanied by an increase of the antioxidant capacity of the body, resulting in a redox status imbalance.23,24The nuclear factor erythroid 2-related factor 2 (Nrf2) is an important transcriptional activator for antioxidant genes and produces anti-inflammatory effects through modulation of nuclear factor-kappa B (NF-κB), a transcription factor that regulates the transcription of genes encoding pro-inflammatory cytokines. The impaired Nrf2 and NF-κB system contributes to increased oxidative stress and systemic inflammation in CKD.25 As partners in crime, oxidative stress and inflammation are highly intertwined processes, and believed to be major causes of CKD progression and complications associated with CKD such as CVD.4,23
CKD is also associated with gut dysbiosis and increased gut barrier permeability, due to CKD-associated factors such as uremia, pharmacological therapies and dietary restrictions.26 A remarkable association between CKD and gastrointestinal dysregulation has recently been reported: 5/6 nephrectomy in mice altered gastrointestinal motility and caused constipation by changing the gut microbiota and causing colonic inflammation.27 Gut dysbiosis is believed to contribute to CVD, because uremic toxins produced by the altered gut microbiota, such as indoxyl sulfate (IS), p-cresyl sulfate (p-CS), trimethylamine N-oxide (TMAO) and indole-3-acetic acid (IAA), accumulate in CKD patients, playing a role in oxidative stress and inflammation.28 Moreover, these toxins also lead to increased ROS production, activate NADPH oxidase and induce an increase in inflammatory cytokines.28,29 Recently, a study showed that elevated levels of uremic toxins in hemodialysis patients were associated positively with NF-κB expression and negatively with Nrf2.30 Uremic toxins are known to exert a wide range of toxic activities such as promoting endothelial dysfunction, and are independently associated with CVD in CKD patients.31,32 Additionally, dysbiosis leads to increased production of lipopolysaccharides (LPS), a major component of the outer membrane of Gram-negative bacteria, and the disrupted intestinal barrier contributes to the translocation of LPS into the host's internal environment, activating inflammation.33
Recently Yu et al.34 showed that intestinal inflammation (reflected by the increase of TNF, IL-6 and IL-10 in the gut) increased in nephrectomized rats compared to a control group, and that increased iNOS appeared to play an important role in the induction of inflammation. In another study, nephrectomized rats showed a depletion of epithelial narrow junction proteins in the gut, significant reductions of Nrf2 and its target genes (NQO1, catalase and CuZn SOD), and activation of NF-κB with increased expression of inflammatory molecules (COX-2, MCP-1, iNOS and gp91phox).35
Although lifestyle modifications, pharmacological interventions, and optimization of dialysis may improve the risk factor profile in CKD, no dramatic improvements in life expectancy in this high-risk patient group have been documented.2 A Western type diet with high consumption of salt and animal proteins has been linked to progression of CKD, showing a strong correlation between CKD and food intake.36 Since interventions with bioactive compounds may modulate inflammation, oxidative stress and dysbiosis, improving quality of life and survival in CKD patients,37–39 dietary therapies may be an alternative way to attenuate the uremic risk factor profile. Among many bioactive compounds, those present in fruits such as berries, with intense red coloration, are thought to be beneficial.37
Cranberry
American cranberry (Vaccinium macrocarpon Ait.) is a member of the Ericaceae family, originally from New England, which since long time has been used in traditional medicine11 and recently it has also been used as a dietary supplement to improve renal failure in animals (felines).12Cranberry is unique among fruits because it has a relatively low natural carbohydrate content compared with its high contents of vitamins, minerals and polyphenolic compounds including flavanols, anthocyanins, proanthocyanidins (PACs), phenolic acids and terpenes, which confer potent antioxidant activity to cranberry.11,15,40 There is growing evidence that the phytochemicals present in cranberry differentiate them from other fruits and contribute to some of their benefits.41
PACs are formed by polymerization of flavan-3-ol units and characterized by epicatechin tetra- and pentamers and are part of the phenylpropanol metabolism leading to the synthesis of anthocyanins. Among PACs, the interflavonoid type A binding (PAC type A) represents about 95% of the PACs present in cranberry.42,43 PACs may protect against bacterial infections by inhibiting the adhesion of bacteria, especially Escherichia coli, to the epithelial cells of the urinary tract.43–46
Based on these observations, cranberry juice has been used for many years to treat symptoms and to prevent recurrent urinary tract infections (UTI) by inhibiting bacteria from sticking to the walls of the bladder. However, a systematic review in 2012 concluded that cranberry juice was less effective in the prevention of UTIs than previously indicated.47–49 Furthermore, it was reported that, among several other non-antibiotic measures of UTI prevention, the quality of studies testing cranberry juice to prevent UTI varies considerably.50,51
In addition to potential urinary antibacterial effects, cranberry PACs are thought to lower the risk of dental caries, periodontitis, CVD and gastrointestinal diseases caused by Helicobacter pylori and norovirus.11
The bioavailability of PACs is not yet clear in the current literature.52–54In vitro models show that PACs are degraded in the stomach due to the release of acid substances. However, studies in humans have shown that PACs may remain stable until they reach the small intestine. An explanation for this is that the acid secretion in the stomach is buffered by the bolus, which leads to a lower exposure of the PACs to the acid medium.55,56 In the small intestine, PACs are poorly absorbed due to their polymeric structure and high molecular weight which results in high concentrations in the colon. It is suggested that PACs are metabolized in the colon through various reactions mediated by esterase, glycosidase, demethylation, dehydroxylation activities and decarboxylation of bacteria, generating products such as phenolic acids and valerolactones,57 which are absorbed in the intestinal mucosa promoting health benefits to the body such as antioxidant and anti-inflammatory effects.58,59 At the same time, polyphenols and their metabolites can affect the intestinal ecology modulating the microbiota.38
Ou et al.60 have in vitro demonstrated that most of the ingested PACs, both type B and type A, are degraded by the gut microbiota into various phenolic compounds, including benzoic acid, 2-phenylacetic acid, 3-phenylpropionic acid, 2-(3-hydroxyphenyl) acetic acid, 2-(4-hydroxyphenyl) acetic acid, 3-(3-hydroxyphenyl) propionic acid and hydroxyphenylvaleric acid. However, it is not clear how much this bacterial metabolism may contribute to the pool of circulating flavanol metabolites.61
McKay et al. (2015)62 were the first to observe that cranberry bioactives, particularly PAC-A2, were detectable in plasma and quantifiable in urine in 10 healthy older adults over a 24 h period after the intake of a low-calorie cranberry juice cocktail (54% juice). In 2016, Feliciano et al.63 observed that, after consumption of a cranberry juice (787 mg polyphenols) in healthy young men, the most abundant plasma metabolites were hippuric acid, catechol-O-sulfate, 2,3-dihydroxybenzoic acid, phenylacetic acid, isoferulic acid, 4-methylcatechol-O-sulfate, α-hydroxyhippuric acid, ferulic acid 4-O-sulfate, benzoic acid, 4-hydroxyphenyl acetic acid, dihydrocaffeic acid 3-O-sulfate, and vanillic acid-4-O-sulfate.
In another study, Feliciano et al.64 observed that there was a linear dose-dependent increase of metabolites after consumption of cranberry juice, although with large inter-individual variation, and that the most abundant plasma metabolites were 3-(4-hydroxyphenyl) propionic acid, hippuric acid, and catechol-O-sulfate. The authors attributed the large inter-individual variation to inter-individual differences in gut microbiota profiles.
Cranberry: effects on gut microbiota
The human body harbors ≈100 trillion intestinal microorganisms, which play an important physiological role in the metabolism and other functions of the host and play a critical role in human health. Given the antimicrobial action of cranberries in the urinary tract, the putative effects of cranberries, and how polyphenols modulate the composition of the gut microbiota, need to be uncovered.65Polyphenols, those present in cranberry, may promote alterations in the composition of the gut microbiota through selective prebiotic effects and antimicrobial activities.66 Additionally, they may reduce inflammation and improve the morphology and homeostasis of the mucosal layer of the intestine.52
In a study on rats using enteral nutrition, cranberry reversed the impaired intestinal barrier by improving the gut mucus layer morphology and function through stimulating the increase of the size and number of goblet cells, as well as increasing the levels of mucin 2, IL-4 and IL-13.67 Mucins are secreted by goblet cells (specialized intestinal epithelial cells) and play a critical role in maintaining mucosal integrity. Goblet cells migrate up the villi after differentiating from crypt stem cells, turning over with the epithelial layer every 3–5 days. Mucin secretion is induced by cholinergic stimulation, while its production is regulated by the T-helper 2 cytokines IL-4 and IL-13, derived from lamina or intraepithelial lymphocytes.68
Anhê et al.69 observed that cranberry administration improved insulin sensitivity, decreased triglycerides, and alleviated intestinal inflammation and oxidative stress in mice that received high fat/high sucrose feeding. The authors also suggested that cranberry was able to stimulate the growth of the Akkermansia sp, a Gram-negative strict anaerobe and mucin-degrading bacterium that has been linked to the protection of the intestinal barrier.
Cranberry: inflammation, oxidative stress and cardiovascular disease
Most of the lifestyle-related chronic diseases that are associated with increasing age are characterized by low-grade inflammation (“inflammaging”) and oxidative stress, conditions that contribute to an increased cardiovascular risk.35–37The polyphenols present in cranberry are considered to be potent antioxidants and the majority of studies have been focused on this property.70–72 The exact mechanisms for such actions are not known, but it can be speculated that polyphenols neutralize ROS and interfere with cell signaling pathways.73,74
Thus, polyphenols modulate the expression of critical genes in the process of stress response, inducing an increased endogenous antioxidant response.75 Cranberries have demonstrated effects on surrogate markers of inflammation and oxidative stress in animal studies, in vitro studies, and even in clinical studies in non-CKD patients (Table 1). It has been hypothesized that these effects result from the combined action of NF-κB inhibition and Nrf2 activation.13,35
| References | Sample/study | Intervention | Results |
|---|---|---|---|
| Abbreviations: AOPP: advanced oxidation protein products; ATP: adenosine triphosphate; COX2: cyclo-oxygenase-2; CRP: C-reactive protein; FRAP: ferric reducing antioxidant power; GPx: glutathione peroxidase; GSH: reduced glutathione; HDL: high density lipoprotein; HOMA-IR: homeostatic model assessment of insulin resistance; IL-6: interleukin-6; IL-4: interleukin 4; LDL: low density lipoprotein; oxidized low-density lipoprotein (oxLDL); LPS: lipopolysaccharides; MDA: malondialdehyde; NAFLD: non-alcoholic fatty liver disease; NF-κB: nuclear factor κB; RCT: randomized clinical trial; SOD: superoxide dismutase; TG: triglyceride; TNF-α: tumor necrosis factor-α; VUR: vesicoureteric reflux; PAC: pro-anthocyanidins; PGE2: prostaglandin E2; PGC-1α: peroxisome proliferator-activated receptor gamma coactivator 1-alpha; TLR-4: toll-like receptor 4. | |||
| Animal studies | |||
| Han et al. (2007)71 | 32 rabbits with oxidative renal damage induced by VUR infection | 1 g kg−1 d−1 of cranberry powder for 3 weeks | Mild mononuclear cell infiltration with no interstitial fibrosis |
| Kim et al. (2013)72 | 40 obese diabetic homogeneous C57BL/KsJ-db/db | – Freeze dried cranberry – anthocyanin (120 mg per 100 g d−1) and PAC (2600 mg per 100 g d−1) for 6 weeks | ↓CRP levels, IL-1β and IL-6 levels |
| ↑HDL | |||
| Pierre et al. (2013)67 | 70 mice | Group A: 8 mg d−1 of PAC for 5 d | Groups A and B: ↑IL-13 |
| Group B: 50 mg d−1 of PAC for 5 d | Group C: ↑IL-4 and IL-13 | ||
| Group C:100 mg d−1 of PAC for 5 d | |||
| Kim et al. (2014)14 | 40 male Sprague-Dawley rats | – Freeze dried cranberry – anthocyanin (120 mg per 100 g d−1) and PAC (2600 mg per 100 g d−1) for 6 weeks. | ↓Catalase activity and SOD |
| Prevent LPS-induced oxidative stress | |||
| Anhê et al. (2015)79 | 36 C57BL/6J mice | 200 mg kg−1 d−1 of cranberry extract for 8 weeks | ↓Liver weight and TG accumulation |
| ↓Intestinal TG content | |||
| ↑Insulin sensitivity | |||
| Peixoto et al. (2018)77 | 72 male Wistar rats | 200 mg kg−1 d−1 of cranberry extract for 4 weeks | ↓TG; ↓hepatic cholesterol and fatty acid synthase contents |
| ↓OxLDL; ↓protein carbonylation | |||
| ↓Accumulation of liver fat | |||
| Glisan et al. (2016)76 | 48 obese rats | Polyphenol-rich cranberry extract 0.8%/d for 10 weeks | ↓ALT and histological severity of NAFLD; ↓hepatic protein levels of TNF-α and C–C chemokine ligand 2; ↓hepatic mRNA levels of TLR-4 and NF-κB |
| Galal et al. (2018)90 | 40 rats; hepatorenal damage and cardiotoxicity induced by AlCl3 | Cranberry extract (100 mg per kg body weight per day) for 4 weeks | ↓Urea, creatinine, ALT, AST, TG, cholesterol, LDL, MDA hepatic and renal; ↑FRAP |
| Patient studies | |||
| Simão et al. (2013)40 | 56 individuals with the metabolic syndrome/non-controlled trial | 700 mL d−1 cranberry juice for 8 weeks | ↑Adiponectin and folic acid |
| ↓Homocysteine; ↓OxLDL and protein oxidation levels | |||
| Basu et al. (2011)81 | 31 individuals with the metabolic syndrome/RCT | Low-energy cranberry juice: 480 mL d−1 for 8 weeks | ↑Antioxidant capacity |
| ↓OxLDL | |||
| Ruel et al. (2005)70 | 21 healthy men/Single-arm intervention | Low-energy cranberry juice: 7 ml kg−1 d−1 for 2 weeks | ↑Antioxidant capacity |
| ↓OxLDL | |||
| Duthie et al. (2006)73 | 20 healthy female volunteers/RCT | Cranberry juice: 750 mL d−1 for 2 weeks | ↑Plasma vitamin C |
| Valentova et al. (2007)74 | 65 healthy women/RCT | Dried cranberry juice Encapsulated 400 mg d−1 or 1200 mg d−1 for 8 weeks | ↓AOPP |
| Chew et al. (2019)78 | 78 overweight or obese men and women/RCT | 450 mL d−1 low calorie high polyphenol cranberry extract beverage for 8 weeks | ↓Endothelin-1; ↓CRP; ↓serum insulin; ↓oxidized glutathione ratio |
| ↑Interferon-γ; ↑nitric oxide; ↑HDL cholesterol | |||
| Lee et al. (2008)84 | 30 type 2 diabetic subjects (men and women) on oral glucose-lowering medication | 500 mg d−1 for 12 weeks | ↓Total cholesterol; ↓LDL cholesterol; ↓total: HDL ratio cholesterol |
| Mathison et al. (2014)82 | 12 healthy adults/crossover | Cranberry leaf extract beverage, low-calorie cranberry juice cocktail: 475 mL – acute | ↑GPx activity, GSH concentration, SOD activity; ↓IL-4 |
| Novotny et al. (2015)80 | 56 healthy individuals | 2 bottles of 240 mL d−1 of low-calorie cranberry juice for 8 weeks | ↓TG; ↓fasting plasma glucose |
| Improve HOMA-IR | |||
| Schell et al. (2017)89 | 25 adults with type 2 diabetes | Dried cranberries (40 g d−1) for 2 weeks | ↓Glucose; ↓IL-18 and MDA |
| Skarpańska-Stejnborn et al. (2017)15 | 16 members of the Polish rowing team/RCT | 200 mg d−1 of cranberry extract for 6 weeks | No change |
| Cell studies | |||
| Denis et al. (2015)13 | Intestinal Caco-2/15 cells for 10 days | 250 μg mL−1 d−1 of cranberry extract | ↓Lipid oxidation, PGE2 levels and COX2, TNF-α, IL-6, NF-kB expression |
| ↑SOD activity, GPx activity, catalase activity, PGC-1α expression, ATP production | |||
Currently, there is encouraging, but limited, evidence about the cardioprotective effect of cranberries. This benefit may be mediated by not only antioxidant effects (for example, decreasing lipid peroxidation and protein oxidation), but also other action such as: decreasing homocysteine levels, modulating lipoprotein profiles (increasing HDL-c, apo A-1 and paraoxonase-1 levels and, reducing oxidized LDL and apoB),13,45,76–78 regulating blood pressure (increasing nitric oxide production), promoting more effective uptake of glucose by insulin-sensitive tissues, and reducing the levels of inflammatory biomarkers.79–85
Moreover, studies have shown that consumption of cranberry may decrease epithelial dysfunction by decreasing endothelial progenitor cells.86 Endothelial progenitor cells are involved in continuous vascular injury, through abnormal repair and tissue calcification.86
In a study conducted by Rodriguez-Mateos et al. (2016)86 involving healthy males, cranberry juice was able to improve vascular function increasing flow-mediated dilation in a dose-dependent manner, with maximal effects 4 h after consumption of juice containing 1238 mg total polyphenols. They also observed that twelve polyphenol metabolites measured in the plasma significantly correlated with the increases in flow-mediated dilation. Dohadwala et al. (2011),87 in a study involving subjects with coronary heart disease, also observed acute effects of cranberry juice containing 835 mg of total polyphenols improving flow-mediated dilation. However, no chronic effect on measures of endothelial vasodilator function was found, agreeing with the study of Flammer et al. (2013)88 which did not report any chronic effect on vascular function after cranberry juice intervention (2 × 230 ml day−1 for four months containing 1740 μg ml−1 of total phenolics) in individuals with peripheral endothelial dysfunction and cardiovascular risk factors.
In addition, the metabolic effects of cranberries could also confer benefits to individuals with the metabolic syndrome and diabetes mellitus.76,77 In fact, Schell et al.89 showed that addition of dried cranberries to a high fat meal improved postprandial blood glucose in obese patients with type 2 diabetes. Cranberries seem to be effective also in decreasing hepatic steatosis, fatty acid synthesis, TNF levels, and mRNA levels of toll-like receptor 4 and NF-kB in the liver.76,77,86
Galal et al.90 demonstrated protective effects of a cranberry extract (and losartan) on hepatorenal damage and cardiotoxicity in mice. Cranberry was able to reduce the concentrations of creatinine and urea. To the best of our knowledge, there are not yet any studies in which the effects of cranberries have been tested in the context of CKD.
Cranberry: cellular senescence and ageing
Cellular senescence, which is a process that results in permanent proliferative arrest on cells in response to various triggers, has emerged as an important contributor to ageing as such and age-related diseases.91 In CKD, chronic low-grade inflammation,92 macromolecular and organelle dysfunction, and cellular senescence are linked to systemic ageing phenotypes,20 as well as to the localized pathogenesis. For example, an increased number of p16INK4a and SA-β-Gal positive senescent cells observed in human uremic arteries were parallelly correlated with the extent of vascular calcification, a typical phenotype of early vascular ageing.93,94 Abundant pre-clinical studies have demonstrated that senotherapy could alleviate multiple phenotypes in progeric mice, biologically aged mice and mice with age-related diseases.95–98 Thus, targeting cellular senescence could be a promising approach to delay, alleviate or prevent the fundamental progression of CKD and senescence-associated complications.97Polyphenols as part of cranberry bioactive compounds are divided into flavonoid and non-flavonoid compounds, and, as mentioned above, exhibit antioxidant, anti-inflammatory and anti-microbial activities. Importantly, some flavonoids and non-flavonoids have been further investigated for their effect on senescent cells and senescence-associated secretary phenotypes (SASP). Though not fully elucidated, growing evidence has shown that sirtuin is a critical factor in modulating cellular senescence through the suppression of telomere attrition and the activation of DNA damage repair.98 Rahnasto-Rilla et al.99 recently evaluated the role of a large array of natural flavonoids as modulators of SIRT6 and showed that different classes of flavonoids can either inhibit or activate SIRT6 deacetylation activity in vitro and, interestingly, the most prominent activators for SIRT6 deacetylation among the flavonoids were anthocyanidins, an abundant bioactive compound in cranberries. Moreover, the therapeutic strategy of the activation of sirtuins is linked to the inhibition of the mammalian target of rapamycin (mTOR) signaling. Natural flavonoids such as quercetin could alleviate ageing processes via AMPK through an mTOR dependent mechanism.100,101 Apart from targeting mTOR pathways, Feresin et al.102 reported that polyphenol extracts from blackberry, raspberry and black raspberry can attenuate Ang II-induced ROS levels, as well as SA-β-gal activity and expression of p21 and p53 in VSMCs through Nox1 dependent or Nox1/ROS-independent pathways. Although the polyphenol contents in this study were not directly extracted from cranberries, this could at least implicate that cranberry polyphenols may exert a similar capacity in the inhibition of cellular senescence activities, since it shares a comparable polyphenol profile with these berries.
Also, it is worth noting that the natural flavanol quercetin, as one of the first reported senolytic agents, showed particular promise in selective clearing of senescent cells by inhibiting PI3K, serpine and other anti-apoptotic regulators in senescent HUVECs.103 In a combined treatment with dasatinib, it has been further verified as a compound capable of alleviating physical dysfunction and increasing late-life survival in aged mice.104
Among non-flavonoid extracts from cranberry, caffeic and ferulic acids have been reported to increase the activity of intracellular antioxidant enzymes and inhibit the production of matrix-metalloproteinase-1 (MMP-1), a component of SASP, in immortalized human keratinocytes upon ultraviolet-A exposure.105
Summary and conclusion
Chronic kidney disease and other chronic diseases that largely may be the consequence of lifestyle disorders, such as obesity, diabetes, osteoporosis, depression and coronary heart disease, are manifested by inflammaging and Nrf2 downregulation within the Nrf2 diseasome.106 In addition, patients with CKD frequently suffer from gut dysbiosis contributing to a pro-oxidant and pro-inflammatory milieu, which may contribute to CKD progression and enhanced cardiovascular risk. These observations suggest that inflammation, oxidative stress and gut dysbiosis should be logical targets for interventions in CKD patients. There is growing evidence that nutritional strategies may reduce some of these complications. Considering that cranberry is a rich source of proanthocyanidins (PACs) – the main bioactive compound found in cranberries – this fruit may alleviate oxidative stress, inflammation and gut dysbiosis, suggesting that supplementation with cranberries should be considered. We suggest that there is now ample background information to contemplate studies aiming at testing if regular cranberry intake could confer benefits to CKD patients (Fig. 1). Although no studies have yet tested the effects of cranberry supplementation in CKD patients, based on studies discussed in this review, there are reasons to hope that cranberries could have beneficial metabolic effects, alleviating oxidative stress, inflammation and gut dysbiosis in CKD patients.Financial support
Conselho Nacional de Pesquisa (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) support Denise Mafra research. The Heart and Lung Foundation, CIMED and “Njurfonden” support Peter Stenvinkel's research. Baxter Novum is the result of a grant from Baxter Healthcare to Karolinska Institutet. Bengt Lindholm is affiliated with Baxter Healthcare.Conflicts of interest
There are no conflicts of interest to declare.References
- A. Levin, M. Tonelli and J. Bonventre, et al., Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy, Lancet, 2017, 390, 1888–1917 CrossRef.
- P. Stenvinkel, Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease, J. Intern. Med., 2010, 268, 456–467 CrossRef CAS PubMed.
- A. C. Webster, E. V. Nagler and R. L. Morton, et al., Chronic Kidney Disease, Lancet, 2017, 389, 1238–1252 CrossRef.
- B. P. Oberg, E. McMenamin and F. L. Lucas, et al., Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease, Kidney Int., 2004, 65, 1009–1016 CrossRef PubMed.
- Z. A. Massy, P. Stenvinkel and T. B. Drueke, The role of oxidative stress in chronic kidney disease, Semin. Dial., 2009, 22, 405–408 CrossRef PubMed.
- W. L. Lau, K. Kalantar-Zadeh and N. D. Vaziri, The gut as a source of inflammation in chronic kidney disease, Nephron, 2015, 130, 92–98 CrossRef CAS PubMed.
- N. A. Borges, F. L. Carmo and M. B. Stockler-Pinto, et al., Probiotic Supplementation in Chronic Kidney Disease: A Double-blind, Randomized, Placebo-controlled Trial, J. Renal Nutr., 2018, 28, 28–36 CrossRef CAS PubMed.
- M. Esgalhado, P. Stenvinkel and D. Mafra, Nonpharmacologic Strategies to Modulate Nuclear Factor Erythroid 2-related Factor 2 Pathway in Chronic Kidney Disease, J. Renal Nutr., 2017, 27, 282–291 CrossRef CAS.
- K. Kowalska, A. Olejnik and D. Szwajgier, et al., Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3 T3-L1 adipose cells, PLoS One, 2017, 12, e0188583 CrossRef PubMed.
- J. Rysz, B. Franczyk and A. Cialkowska-Rysz, et al., The Effect of Diet on the Survival of Patients with Chronic Kidney Disease, Nutrients, 2017, 9, e495 CrossRef PubMed.
- S. Zhao, H. Liu and L. Gu, American cranberries and health benefits - an evolving story of 25 years, J. Sci. Food Agric., 2018 DOI:10.1002/jsfa.8882.
- A. D. Cerbo, T. Iannitti and G. Guidetti, et al., A nutraceutical diet based on Lespedeza spp., Vaccinium macrocarpon and Taraxacum officinale improves spontaneous feline chronic kidney disease, Physiol. Rep., 2018, 6, e13737 CrossRef PubMed.
- M. C. Denis, Y. Desjardins and A. Furtos, et al., Prevention of oxidative stress, inflammation and mitochondrial dysfunction in the intestine by different cranberry phenolic fractions, Clin. Sci., 2015, 128, 197–212 CrossRef CAS.
- M. J. Kim, J. H. Kim and H. K. Kwak, Antioxidant effects of cranberry powder in lipopolysaccharide treated hypercholesterolemic rats, Prev. Nutr. Food Sci., 2014, 19, 75–81 CrossRef CAS PubMed.
- A. Skarpanska-Stejnborn, P. Basta and J. Trzeciak, et al., Effects of cranberry (Vaccinum macrocarpon) supplementation on iron status and inflammatory markers in rowers, J. Int. Soc. Sports Nutr., 2017, 14, 7 CrossRef PubMed.
- M. Juthani-Mehta, P. H. Van Ness and L. Bianco, et al., Effect of Cranberry Capsules on Bacteriuria Plus Pyuria Among Older Women in Nursing Homes: A Randomized Clinical Trial, J. Am. Med. Assoc., 2016, 316, 1879–1887 CrossRef CAS PubMed.
- A. Ledda, A. Bottari and R. Luzzi, et al., Cranberry supplementation in the prevention of non-severe lower urinary tract infections: a pilot study, Eur. Rev. Med. Pharmacol. Sci., 2015, 19, 77–80 CAS.
- A. Luis, F. Domingues and L. Pereira, Can Cranberries Contribute to Reduce the Incidence of Urinary Tract Infections? A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Clinical Trials, J. Urol., 2017, 198, 614–621 CrossRef.
- K. C. Maki, K. L. Kaspar and C. Khoo, et al., Consumption of a cranberry juice beverage lowered the number of clinical urinary tract infection episodes in women with a recent history of urinary tract infection, Am. J. Clin. Nutr., 2016, 103, 1434–1442 CrossRef CAS PubMed.
- J. P. Kooman, P. Kotanko and A. M. Schols, et al., Chronic kidney disease and premature ageing, Nature, 2014, 10, 732–742 CAS.
- A. G. Miranda-Diaz, L. Pazarin-Villasenor and F. G. Yanowsky-Escatell, et al., Oxidative Stress in Diabetic Nephropathy with Early Chronic Kidney Disease, J. Diabetes Res., 2016, 2016, 7047238 Search PubMed.
- P. S. Tucker, A. T. Scanlan and V. J. Dalbo, Chronic kidney disease influences multiple systems: describing the relationship between oxidative stress, inflammation, kidney damage, and concomitant disease, Oxid. Med. Cell. Longevity, 2015, 2015, 806358 Search PubMed.
- T. Antunovic, A. Stefanovic and N. B. Gligorovic, et al., Prooxidant-antioxidant balance, hsTnI and hsCRP: mortality prediction in haemodialysis patients, two-year follow-up, Renal failure, 2017, 39, 491–499 CrossRef CAS.
- N. Krata, R. Zagozdzon and B. Foroncewicz, et al., Oxidative Stress in Kidney Diseases: The Cause or the Consequence?, Arch. Immunol. Ther. Exp., 2018, 66, 211–220 CrossRef CAS PubMed.
- H. J. Kim and N. D. Vaziri, Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure, Am. J. Physiol.: Renal. Physiol., 2010, 298, F662–F671 CrossRef CAS.
- W. L. Lau and N. D. Vaziri, The Leaky Gut and Altered Microbiome in Chronic Kidney Disease, J. Renal Nutr., 2017, 27, 458–461 CrossRef CAS PubMed.
- K. Nishiyama, K. Aono and Y. Fujimoto, et al., Chronic kidney disease after 5/6 nephrectomy disturbs the intestinal microbiota and alters intestinal motility, J. Cell. Physiol., 2018, 234, 6667–6678 CrossRef PubMed.
- D. Mafra, J. C. Lobo and A. F. Barros, et al., Role of altered intestinal microbiota in systemic inflammation and cardiovascular disease in chronic kidney disease, Future Microbiol., 2014, 9, 399–410 CrossRef CAS PubMed.
- L. Simoes-Silva, R. Araujo and M. Pestana, et al., The microbiome in chronic kidney disease patients undergoing hemodialysis and peritoneal dialysis, Pharmacol. Res., 2018, 130, 143–151 CrossRef CAS PubMed.
- M. B. Stockler-Pinto, C. O. Soulage and N. A. Borges, et al., From bench to the hemodialysis clinic: protein-bound uremic toxins modulate NF-kappaB/Nrf2 expression, Int. Urol. Nephrol., 2018, 50, 347–354 CrossRef CAS.
- R. Poesen, K. Claes and P. Evenepoel, et al., Microbiota-Derived Phenylacetylglutamine Associates with Overall Mortality and Cardiovascular Disease in Patients with CKD, J. Am. Soc. Nephrol., 2016, 27, 3479–3487 CrossRef CAS.
- H. Y. Kim, T. H. Yoo and Y. Hwang, et al., Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD), Sci. Rep., 2017, 7, 3057 CrossRef PubMed.
- L. Koppe, D. Mafra and D. Fouque, Probiotics and chronic kidney disease, Kidney Int., 2015, 88, 958–966 CrossRef CAS PubMed.
- C. Yu, S. Tan and Z. Wang, et al., Chronic Kidney Disease Elicits an Intestinal Inflammation Resulting in Intestinal Dysmotility Associated with the Activation of Inducible Nitric Oxide Synthesis in Rat, Digestion, 2018, 97, 205–211 CAS.
- W. L. Lau, S. M. Liu and S. Pahlevan, et al., Role of Nrf2 dysfunction in uremia-associated intestinal inflammation and epithelial barrier disruption, Dig. Dis Sci., 2015, 60, 1215–1222 CrossRef CAS PubMed.
- H. Kramer, Kidney Disease and the Westernization and Industrialization of Food, Am. J. Kidney Dis., 2017, 70, 111–121 CrossRef PubMed.
- L. F. Cardozo, L. M. Pedruzzi and P. Stenvinkel, et al., Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2, Biochimie, 2013, 95, 1525–1533 CrossRef CAS PubMed.
- I. Martins, N. A. Borges and P. Stenvinkel, et al., The value of the Brazilian acai fruit as a therapeutic nutritional strategy for chronic kidney disease patients, Int. Urol. Nephrol., 2018, 50, 2207–2220 CrossRef CAS PubMed.
- A. D. Cerbo, F. Pezzuto and L. Palmieri, et al., Clinical and experimental use of probiotic formulations for management of end-stage renal disease: an update, Int. Urol. Nephrol., 2013, 45, 1569–1576 CrossRef PubMed.
- T. N. Simão, M. A. Lozovoy and A. N. Simão, et al., Reduced-energy cranberry juice increases folic acid and adiponectin and reduces homocysteine and oxidative stress in patients with the metabolic syndrome, Br. J. Nutr, 2013, 110, 1885–1894 CrossRef PubMed.
- J. B. Blumberg, T. A. Camesano and A. Cassidy, et al., Cranberries and their bioactive constituents in human health, Adv. Nutr., 2013, 4, 618–632 CrossRef CAS PubMed.
- R. P. Feliciano, J. J. Meudt and D. Shanmuganayagam, et al., Ratio of “A-type” to “B-type” proanthocyanidin interflavan bonds affects extra-intestinal pathogenic Escherichia coli invasion of gut epithelial cells, J. Agric. Food Chem., 2014, 62, 3919–3925 CrossRef CAS PubMed.
- L. Y. Foo, Y. Lu and A. B. Howell, et al., A-Type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli, J. Nat. Prod., 2000, 63, 1225–1228 CrossRef CAS.
- C. G. Krueger, J. D. Reed and R. P. Feliciano, et al., Quantifying and characterizing proanthocyanidins in cranberries in relation to urinary tract health, Anal. Bioanal. Chem., 2013, 405, 4385–4395 CrossRef CAS PubMed.
- D. L. McKay and J. B. Blumberg, Cranberries (Vaccinium macrocarpon) and cardiovascular disease risk factors, Nutr. Rev., 2007, 65, 490–502 CrossRef PubMed.
- L. Shi, S. Cao and X. Chen, et al., Proanthocyanidin Synthesis in Chinese Bayberry (Myrica rubra Sieb. et Zucc.) Fruits, Front. Plant Sci., 2018, 9, 212 CrossRef PubMed.
- K. C. Maki, K. M. Nieman and A. L. Schild, et al., The Effect of Cranberry Juice Consumption on the Recurrence of Urinary Tract Infection: Relationship to Baseline Risk Factors, J. Am. Coll. Nutr., 2018, 37, 121–126 CrossRef CAS PubMed.
- A. Shatkin-Margolis, J. Warehime and R. N. Pauls, Cranberry Supplementation Does Not Reduce Urinary Tract Infections in Patients With Indwelling Catheters After Pelvic Reconstructive Surgery, Female Pelvic Med Reconstr Surg, 2018, 24, 130–134 CrossRef PubMed.
- R. G. Jepson, G. Williams and J. C. Craig, Cranberries for preventing urinary tract infections, Cochrane Database Syst Rev, 2012, 10, CD001321 Search PubMed.
- A. K. Gunnarsson, L. Gunningberg and S. Larsson, et al., Cranberry juice concentrate does not significantly decrease the incidence of acquired bacteriuria in female hip fracture patients receiving urine catheter: a double-blind randomized trial, Clin. Interventions Aging, 2017, 12, 137–143 CrossRef CAS PubMed.
- D. Wojnicz, D. Tichaczek-Goska and K. Korzekwa, et al., Study of the impact of cranberry extract on the virulence factors and biofilm formation by Enterococcus faecalis strains isolated from urinary tract infections, Int. J. Food Sci. Nutr., 2016, 67, 1005–1016 CrossRef PubMed.
- A. Scalbert, S. Deprez and I. Mila, et al., Proanthocyanidins and human health: systemic effects and local effects in the gut, Biofactors, 2000, 13, 115–120 CrossRef CAS PubMed.
- G. Peron, A. Pellizzaro and P. Brun, et al., Antiadhesive Activity and Metabolomics Analysis of Rat Urine after Cranberry (Vaccinium macrocarpon Aiton) Administration, J. Agric. Food Chem., 2017, 65, 5657–5667 CrossRef CAS PubMed.
- M. M. Appeldoorn, J. P. Vincken and H. Gruppen, et al., Procyanidin dimers A1, A2, and B2 are absorbed without conjugation or methylation from the small intestine of rats, J. Nutr., 2009, 139, 1469–1473 CrossRef CAS PubMed.
- R. P. Feliciano, C. G. Krueger and J. D. Reed, Methods to determine effects of cranberry proanthocyanidins on extraintestinal infections: Relevance for urinary tract health, Mol. Nutr. Food Res., 2015, 59, 1292–1306 CrossRef CAS PubMed.
- J. P. Spencer, F. Chaudry and A. S. Pannala, et al., Decomposition of cocoa procyanidins in the gastric milieu, Biochem. Biophys. Res. Commun., 2000, 272, 236–241 CrossRef CAS PubMed.
- Y. Y. Choy and A. L. Waterhouse, Proanthocyanidin Metabolism, a mini review, Nutr. Aging, 2014, 2, 111–116 CAS.
- P. C. Karlsson, U. Huss and A. Jenner, et al., Human fecal water inhibits COX-2 in colonic HT-29 cells: role of phenolic compounds, J. Nutr., 2005, 135, 2343–2349 CrossRef CAS PubMed.
- T. Unno, K. Tamemoto and F. Yayabe, et al., Urinary excretion of 5-(3′,4′-dihydroxyphenyl)-gamma-valerolactone, a ring-fission metabolite of (-)-epicatechin, in rats and its in vitro antioxidant activity, J. Agric. Food Chem., 2003, 51, 6893–6898 CrossRef CAS PubMed.
- K. Ou, P. Sarnoski and K. R. Schneider, et al., Microbial catabolism of procyanidins by human gut microbiota, Mol. Nutr. Food Res., 2014, 58, 2196–2205 CrossRef CAS PubMed.
- J. I. Ottaviani, C. Kwik-Uribe and C. L. Keen, et al., Intake of dietary procyanidins does not contribute to the pool of circulating flavanols in humans, Am. J. Clin. Nutr., 2012, 95, 851–858 CrossRef CAS PubMed.
- D. L. McKay, C. Y. Chen and C. A. Zampariello, et al., Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults, Food Chem., 2015, 168, 233–240 CrossRef CAS PubMed.
- R. P. Feliciano, A. Boeres and L. Massacessi, et al., I Identification and quantification of novel cranberry-derived plasma and urinary (poly)phenols, Arch. Biochem. Biophys., 2016, 599, 31–41 CrossRef CAS.
- R. P. Feliciano, C. E. Mills, G. Istas, C. Heiss and A. Rodriguez-Mateos, Absorption, Metabolism and Excretion of Cranberry (Poly)phenols in Humans: A Dose Response Study and Assessment of Inter-Individual Variability, Nutrients, 2017, 9, 268 CrossRef PubMed.
- U. Etxeberria, A. Fernandez-Quintela and F. I. Milagro, et al., Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition, J. Agric. Food Chem., 2013, 61, 9517–9533 CrossRef CAS PubMed.
- F. Cardona, C. Andrés- Lacueva and S. Tulipani, et al., Benefits of polyphenols on gut microbiota and implications in human health, J. Nutr. Biochem., 2013, 24, 1415–1422 CrossRef CAS PubMed.
- J. F. Pierre, A. F. Heneghan and R. P. Feliciano, et al., Cranberry proanthocyanidins improve the gut mucous layer morphology and function in mice receiving elemental enteral nutrition, JPEN, J. Parenter. Enteral. Nutr., 2013, 37, 401–409 CrossRef CAS PubMed.
- K. S. Bergstrom, V. Kissoon-Singh and D. L. Gibson, et al., Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa, PLoS Pathog., 2010, 6, e1000902 CrossRef.
- F. F. Anhê, D. Roy and G. Pilon, et al., A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice, Gut, 2015, 64, 872–883 CrossRef.
- G. Ruel, S. Pomerleau and P. Couture, et al., Changes in Plasma Antioxidant Capacity and Oxidized Low-Density Lipoprotein Levels in Men after Short-Term Cranberry Juice Consumption, Metabolism, 2005, 54, 856–861 CrossRef CAS PubMed.
- C. H. Han, S. Kim and S. H. Kang, et al., Protective effects of cranberries on infection-induced oxidative renal damage in rabbit model of vesico-ureteric reflux, BJU Int., 2007, 100, 1172–1175 Search PubMed.
- M. J. Kim, J. Y. Chung and J. H. Kim, et al., Effects of cranberry powder on biomarkers of oxidative stress and glucose control in db/db mice, Nutr. Res. Pract., 2013, 7, 430–438 CrossRef CAS.
- S. J. Duthie, A. M. Jenkinson and A. Crozier, et al., The Effects of Cranberry Juice Consumption on Antioxidant Status and Biomarkers Relating to Heart Disease and Cancer in Healthy Human Volunteers, Eur. J. Nutr., 2006, 45, 113–122 CrossRef CAS PubMed.
- K. Valentova, D. Stejskal and P. Bednar, et al., Biosafety, antioxidant status, and metabolites in urine after consumption of dried cranberry juice in healthy women: a pilot double-blind placebo-controlled trial, J. Agric. Food Chem., 2007, 55, 3217–3224 CrossRef CAS PubMed.
- A. P. S. Caldas, O. G. L. Coelho and J. Bressan, Cranberry antioxidant power on oxidative stress, inflammation and mitochondrial damage, Int. J. Food Prop., 2018, 21, 582–592 CrossRef CAS.
- S. L. Glisan, C. Ryan and A. P. Neilson, et al., Cranberry extract attenuates hepatic inflammation in high-fat-fed obese mice, J. Nutr. Biochem., 2016, 37, 60–66 CrossRef CAS PubMed.
- T. C. Peixoto, E. G. Moura and E. de Oliveira, et al., Cranberry (Vaccinium macrocarpon) extract treatment improves triglyceridemia, liver cholesterol, liver steatosis, oxidative damage and corticosteronemia in rats rendered obese by high fat diet, Eur. J. Nutr., 2018, 57, 1829–1844 CrossRef.
- B. Chew, B. Mathison and L. Kimble, et al., Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: a randomized controlled trial, Eur. J. Nutr., 2019, 58, 1223–1235 CrossRef CAS.
- F. F. Anhê, D. Roy and G. Pilon, et al., A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice, Gut, 2015, 64, 872–883 CrossRef PubMed.
- J. A. Novotny, D. J. Baer and C. Khoo, et al., Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults, J. Nutr., 2015, 145, 1185–1193 CrossRef CAS.
- A. Basu, N. M. Betts and J. Ortiz, et al., Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome, Nutr. Res., 2011, 31, 190–196 CrossRef CAS.
- B. D. Mathison, L. L. Kimble and K. L. Kaspar, et al., Consumption of cranberry beverage improved endogenous antioxidant status and protected against bacteria adhesion in healthy humans: a randomized controlled trial, Nutr. Res., 2014, 34, 420–427 CrossRef CAS PubMed.
- J. B. Blumberg, A. Basu and C. G. Krueger, et al., Impact of Cranberries on Gut Microbiota and Cardiometabolic Health: Proceedings of the Cranberry Health Research Conference 2015, Adv. Nutr., 2016, 7, 759S–770S CrossRef PubMed.
- I. T. Lee, Y. C. Chan and C. W. Lin, et al., Effect of cranberry extracts on lipid profiles in subjects with Type 2 diabetes, Diabetic Med., 2008, 25, 1473–1477 CrossRef CAS PubMed.
- M. M. Dohadwala, M. Holbrook and N. M. Hamburg, et al., Effects of cranberry juice consumption on vascular function in patients with coronary artery disease, Am. J. Clin. Nutr., 2011, 93, 934–940 CrossRef CAS.
- A. Rodriguez-Mateos, R. P. Feliciano and A. W. Boeres, et al., Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study, Mol. Nutr. Food Res., 2016, 60, 2130–2140 CrossRef CAS.
- M. M. Dohadwala, M. Holbrook and N. M. Hamburg, et al., Effects of cranberry juice consumption on vascular function in patients with coronary artery disease, Am. J. Clin. Nutr., 2011, 93, 934–940 CrossRef CAS PubMed.
- A. J. Flammer, E. A. Martin and M. Gossl, et al., Polyphenol-rich cranberry juice has a neutral effect on endothelial function but decreases the fraction of osteocalcin-expressing endothelial progenitor cells, Eur. J. Nutr., 2013, 52, 289–296 CrossRef CAS.
- J. Schell, N. M. Betts and M. Foster, et al., Cranberries improve postprandial glucose excursions in type 2 diabetes, Food Funct., 2017, 8, 3083–3090 RSC.
- S. M. Galal, H. F. Hasan and M. K. Abdel-Rafei, et al., Synergistic effect of cranberry extract and losartan against aluminium chloride-induced hepatorenal damage associated cardiomyopathy in rats, Arch. Physiol. Biochem., 2018, 23, 1–10 CrossRef.
- T. Tchkonia, Y. Zhu and J. van Deursen, et al., Cellular senescence and the senescent secretory phenotype: therapeutic opportunities, J. Clin. Invest., 2013, 123, 966–972 CrossRef CAS PubMed.
- J. P. Kooman, M. J. Dekker and L. A. Usvyat, et al., Inflammation and premature aging in advanced chronic kidney disease, Am. J. Physiol.: Renal, Physiol., 2017, 313, F938–F950 CrossRef.
- P. Stenvinkel, K. Luttropp and D. McGuinness, et al., CDKN2A/p16INK4(a) expression is associated with vascular progeria in chronic kidney disease, Aging Cell, 2017, 9, 494–507 CAS.
- D. J. Baker, T. Wijshake and T. Tchkonia, et al., Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders, Nature, 2011, 479, 232–236 CrossRef CAS PubMed.
- C. M. Roos, B. Zhang and A. K. Palmer, et al., Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice, Aging Cell, 2016, 15, 973–977 CrossRef CAS.
- M. J. Schafer, T. A. White and K. Iijima, et al., Cellular senescence mediates fibrotic pulmonary disease, Nat. Commun., 2017, 8, 14532 CrossRef CAS.
- J. L. Kirkland and T. Tchkonia, Cellular Senescence: A Translational Perspective, EBioMed., 2017, 21, 21–28 Search PubMed.
- S. H. Lee, J. H. Lee and H. Y. Lee, et al., Sirtuin signaling in cellular senescence and aging, BMB Rep., 2019, 52, 24–34 CrossRef CAS.
- M. Rahnasto-Rilla, M. Tyni and J. Huovinen, et al., Natural polyphenols as sirtuin 6 modulators, Sci. Rep., 2018, 8, 4163 CrossRef PubMed.
- P. Stenvinkel, J. P. Kooman and P. G. Shiels, Nutrients and ageing: what can we learn about ageing interactions from animal biology?, Curr. Opin. Clin. Nutr. Metab. Care, 2016, 19, 19–25 CrossRef CAS PubMed.
- J. T. Hwang, D. Y. Kwon and S. H. Yoon, AMP-activated protein kinase: a potential target for the diseases prevention by natural occurring polyphenols, Nat. Biotechnol., 2009, 26, 17–22 CAS.
- R. G. Feresin, J. Huang and D. S. Klarich, et al., Blackberry, raspberry and black raspberry polyphenol extracts attenuate angiotensin II-induced senescence in vascular smooth muscle cells, Food Funct., 2016, 7, 4175–4187 RSC.
- Y. Zhu, T. Tchkonia and T. Pirtskhalava, et al., The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs, Aging Cell, 2015, 14, 644–658 CrossRef CAS.
- M. Xu, T. Pirtskhalava and J. N. Farr, et al., Senolytics improve physical function and increase lifespan in old age, Nat. Med., 2018, 24, 1246–1256 CrossRef CAS PubMed.
- T. Pluemsamran, T. Onkoksoong and U. Panich, Caffeic acid and ferulic acid inhibit UVA-induced matrix metalloproteinase-1 through regulation of antioxidant defense system in keratinocyte HaCaT cells, Photochem. Photobiol., 2012, 88, 961–968 CrossRef CAS PubMed.
- A. Cuadrado, G. Manda and A. Hassan, et al., Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach, Pharmacol. Rev., 2018, 70, 348–383 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |