Catechin inhibits glycated phosphatidylethanolamine formation by trapping dicarbonyl compounds and forming quinone
Abstract
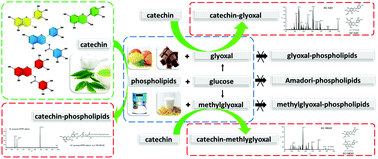
It is important to inhibit food-derived potentially hazardous glycated lipids with natural products. A model reaction inhibition system was established, and products were identified with high-performance liquid chromatography-mass spectrometry (HPLC-MS/MS) to study the inhibitory effects of four types of catechins on the formation of glycated 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE) products. The results show that the percentage inhibition of epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC) and epigallocatechin gallate (EGCG) on the formation of carboxymethyl 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (CM-DPPE) are 38.84%, 33.31%, 20.71% and 22.66%, respectively. The percentage inhibition of EC, ECG, EGC and EGCG on the formation of carboxyethyl 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (CE-DPPE) is 42.04%, 41.99%, 31.70% and 36.24%, respectively. In addition, catechin can capture glyoxal (GO) and methylglyoxal (MGO) to produce multiple products. O-Benzoquinone, the oxidation products of catechin, also captures DPPE to produce quinone–DPPE adducts. Therefore, there are two inhibitory mechanisms of tea-derived catechin for glycated DPPE: (1) catechin inhibits the formation of CM-DPPE and CE-DPPE by trapping reactive GO and MGO; and (2) catechin is oxidized to o-benzoquinone. O-Benzoquinone reacts with DPPE through nucleophilic substitution, which competes with the reaction between glucose and DPPE. This study will provide a theoretical basis for the use of natural products to inhibit the formation of food-derived glycated lipids.


 Please wait while we load your content...
Please wait while we load your content...