Targeted delivery of phycocyanin for the prevention of colon cancer using electrospun fibers†
Abstract
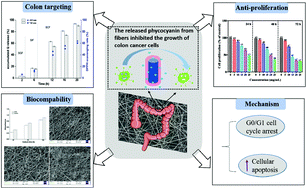
Phycocyanin (PC), a water-soluble biliprotein, exhibits potent anti-colon cancer properties. However, its application in functional foods is limited by the poor stability and low bioavailability of PC. In this study, we successfully encapsulated PC by coaxial electrospinning. The colon targeted release of PC was achieved with retention of the antioxidant activity of PC. The PC-loaded electrospun fiber mat (EFM) obtained inhibited HCT116 cell growth in a dose-dependent and time-dependent manner. In particular, the PC-loaded EFM exerted its anti-cancer activity by blocking the cell cycle at the G0/G1 phase and inducing cell apoptosis involving the decrease of Bcl-2/Bax, activation of caspase 3 and release of cytochrome c. This study suggests that co-axial electrospinning is an efficient and effective way to deliver PC and improve its bioavailability; thus, it represents a promising approach for encapsulating functional ingredients for colon cancer prevention.


 Please wait while we load your content...
Please wait while we load your content...