Open Access Article
Open Access ArticleEffects of probiotic supplementation on the regulation of blood lipid levels in overweight or obese subjects: a meta-analysis†
Shoumeng
Yan
a,
Zhenwei
Tian
b,
Meng
Li
a,
Bo
Li
 *a and
Weiwei
Cui
*c
*a and
Weiwei
Cui
*c
aDepartment of Epidemiology and Biostatistics, School of Public Health, Jilin University, Changchun, 130021, P. R. China. E-mail: tougao1963@163.com; Tel: +86 43185619451
bDepartment of Emergency and Critical Care, The Second Hospital of Jilin University, Jilin University, Changchun, 130021, P. R. China
cDepartment of Nutrition and Food Hygiene, School of Public Health, Jilin University, Changchun, 130021, P. R. China. E-mail: cuiweiwei@jlu.edu.cn; Tel: +86 43185619451
First published on 4th February 2019
Abstract
Background: Obesity is a risk factor for many deadly diseases. Meanwhile, the prevalence of obesity has been continuously increasing in many countries. Probiotics are defined as live microorganisms that confer health benefits on hosts. Probiotic supplementation could reduce body weight, body mass index (BMI) and fat percentage. However, it is unclear whether supplementation with probiotics is beneficial to lower blood lipid levels for obese or overweight people. Methods: In this study, a comprehensive search across multiple databases was performed to identify studies that focused on the effects of probiotics on blood lipid levels in overweight or obese subjects. The meta-analysis included studies that compared the variations in blood lipid (total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL) and triglyceride (TG)) concentrations between overweight and obese subjects who were supplemented with probiotics versus the controls who were not supplemented with probiotics. Results: Our findings indicated that probiotic supplementation in obese or overweight people was associated with significantly larger reductions in TC and LDL levels compared to a lack of probiotic supplementation in the control subjects. However, there was no significant difference in the variations between HDL and TG concentrations. Conclusion: Probiotic supplementation reduced TC and LDL concentrations in obese or overweight people. Additional data from large clinical trials are required to confirm the efficacy and safety of probiotics in the regulation of blood lipid levels in obese or overweight people.
1. Introduction
Obesity is a pathological state marked by the accumulation of excess body mass in the abdominal region as a result of disequilibrium between energy intake and its consumption,1 and it is a risk factor for many deadly diseases, particularly diabetes, cardiovascular disease, non-alcoholic fatty liver disease (NAFLD) and some forms of cancer.2–4 In 2015, 107.7 million (98.7–118.4 million) children and 603.7 million (588.2–619.8 million) adults were obese worldwide. The overall prevalence of obesity in children and adults was 5.0% and 12.0%, respectively. The prevalence of obesity has doubled since 1980 in more than 70 countries and has continuously increased in most other countries.5Probiotics are defined as live microorganisms that confer health benefits on hosts when consumed in appropriate amounts in food.6 This term also refers to some yeasts and bacteria that are used as dietary supplements or additives in certain foods. Previous studies have indicated that probiotics were associated with reducing episodes of diarrhea, malabsorption and dysbiosis.7–9 Probiotic treatment may reduce liver fat, aspartate aminotransferase (AST) level, and glycemic and inflammatory indices in patients with NAFLD.10,11 In addition, probiotics could be an effective option to improve immune function by enhancing natural killer (NK) cell function and interferon (IFN)-γ concentration.12 The use of probiotics also improves the clinical and laboratory profiles of evaluated patients with chronic pancreatitis, favoring the best clinical outcome.13 Moreover, probiotics can effectively prevent colorectal cancer by the alteration of the intestinal microflora, the inactivation of carcinogenic compounds, and the inhibition of tyrosine kinase signaling pathways and other processes.14
Abnormal levels of blood lipids (total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL) and triglyceride (TG)) associated with overweight or obesity are major risk factors for cardiovascular disease.15 Overweight and obese people had higher short chain fatty acids (SCFAs) that were correlated with the development of obesity.16 Meanwhile, intestinal microflora have a suppressive effect on adenosine monophosphate kinase (AMPK) activity, thus affecting fatty acid oxidation and metabolism.1 What's more, probiotics such as lactic acid bacteria could change the gut microbiota community.17 Therefore, probiotics could provide anti-obesity effects in animal models and humans by the regulation of the intestinal microflora.1 Several studies reported that probiotics significantly reduced the TC and LDL levels and improved the HDL concentration.18–20 However, Wong VW found that the use of probiotics was not associated with changes in the body mass index (BMI), waist circumference, glucose and lipid levels.10 The role of probiotics in the blood lipid level of obese or overweight people remains controversial. Therefore, we performed a meta-analysis of all relevant randomized control trials (RCTs) with the main focus on the efficacy of probiotics on the blood lipid levels of obese or overweight people.
2. Materials and methods
2.1. Sources and methods of data retrieval
We performed a comprehensive literature search that included studies from 1970 to September 2018; the electronic databases included PubMed, Medline, Web of Science, Cochrane Library and Google Scholar. We analyzed the variations in TC, LDL, HDL and TG concentrations in the blood lipids of obese or overweight people in response to supplementation with probiotics. The following terms were used for the literature search: probiotics, overweight, obesity, cholesterol, triglyceride, high density lipoprotein, HDL, low density lipoprotein, LDL, and blood lipids. The term ‘OR’ was used as the set operator to combine different sets of results. The literature search was restricted to the English language and human subjects. Location, age, probiotics and other confounding factors were also considered.2.2. Inclusion criteria
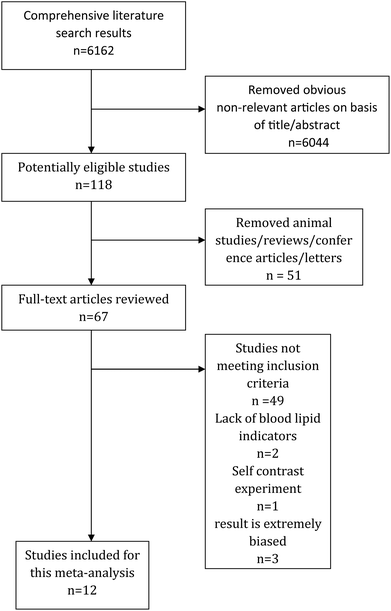
The articles included in this meta-analysis matched the following six criteria: (1) overweight or obesity was defined according to local standards; (2) studies included an intervention group and a control group; (3) the results included quantitative data with specific values; (4) the supplementation groups and the controls had not received probiotic supplementation regularly or efficiently in the past; (5) we excluded subjects who were pregnant or breast feeding; had renal or hepatic dysfunction, heart disease, hypertension, diabetes mellitus, any metabolic disorder, or acute gastrointestinal disorders; or were taking medicines or functional food that may affect the body weight or body fat; and (6) we excluded studies that did not provide initial data, animal studies, in vitro studies, reviews and conference papers. Two investigators independently reviewed the literature, extracted all potentially eligible studies and resolved uncertainty and disagreement by discussion (Fig. 1).2.3. Data abstraction
We reviewed all of the relevant studies and extracted the following data: (1) lead author, nationality, publication year, probiotics, subjects of studies, numbers of patients and controls, mean age and the BMI of the supplementation groups and controls, and gender of the supplementation groups and controls; and (2) the changes in the TC, LDL, HDL and TG concentrations in the supplementation groups and controls.2.4. Risk of bias within individual studies
Two investigators used the Cochrane Collaboration (RevMan Version 5.3) software to evaluate the risk of bias (including the risks of selection bias, performance bias, detection bias, attrition bias, reporting bias and other biases) within the individual studies independently and resolved inconsistencies by discussion and consensus.212.5. Statistical analysis
Statistical analysis was conducted using the statistical software RevMan version 5.3 and Stata (version 12.0, StataCorp LLC, College Station, TX, USA). The mean change (standard deviation) in TC, LDL, HDL and TG levels from the baseline was used to calculate the mean difference (95% confidence interval [CI]) between the intervention group and the control group. When it was not provided by the study's authors, we calculated the standard deviation in the mean change in the inflammatory markers using the formula in the Cochrane handbook.22 The correlation coefficient of the equation was imputed using the data from the included studies reporting the baseline and endpoint values and the variations. Our estimated value of 0.88 indicated that the correlation between the baseline and final values of TC, LDL, HDL and TG was high.We combined the weighted mean difference (WMD) for studies that listed the mean and standard deviation values for the variations in the TC, LDL, HDL and TG concentrations in the intervention and control groups. The fixed effects model and the random effects model were used to determine the WMD and 95% confidence intervals (CIs) and to evaluate the differences in the variations in the blood lipid concentrations between the probiotic supplementation group and the controls.
Cochran's Q statistic and the I2 statistic were used to assess the statistical heterogeneity in the meta-analysis.23 If the data were homogeneous (p > 0.05), a fixed effect model meta-analysis was performed; if the data were heterogeneous (p ≤ 0.05), a random effects model meta-analysis was performed. In the Q test, p < 0.05 was considered significant for heterogeneity, and the I2 value was used to evaluate the degree of heterogeneity. I2 values of 25%, 50% and 75% indicate low, moderate and high heterogeneity, respectively.24 Heterogeneity was analyzed via sensitivity analysis. Subgroup analyses were performed based on the object (adults, children/adolescents, and women), region (Asia, Europe and others), number of probiotics (single and multiple) and probiotic species (Lactobacillus (L), Lactobacillus + Streptococcus (L + S) and Lactobacillus + Streptococcus + Bifidobacterium (L + S + B)).
3. Results
Our study identified 6162 related references, but only 12 papers met our inclusion criteria. These 12 articles included a total of 767 samples, with 391 treatments and 376 controls.25–36 The detailed results are shown in Table 1 and Table S1.† Five of these studies were conducted in Asia,25–27,29,30 five were conducted in Europe,28,31–33,36 one in Oceania,35 and one in America.34 The subjects were adults in seven papers,25–28,34–36 children or adolescents in three papers,29–31 and women in two papers.32,33 Five studies included one single species of probiotics,26–28,31,34 while the remaining studies (n = 7) included two or multiple species of probiotics.25,29,30,32,33,35,36 Seven of the studies reported changes and the baseline and final values of TC, LDL, HDL and TG,26–28,30–32,36 whereas five studies reported changes or the baseline and final values of TC, LDL, HDL and TG.25,29,33–35 The basic characteristics of the patients are presented in Table 1. One of the studies was excluded in the analysis of triglycerides because of heterogeneity (after excluding this study,26 the heterogeneity changed from 82.9% (p < 0.001) to 17.0% (p = 0.151)). Additionally, one study was also excluded in the analysis of LDL (after excluding this study,25 the heterogeneity changed from 61.6% (p = 0.002) to 36.9% (p = 0.096)).| Author | Region | Year | Probiotics | Intervention dose | Subjects | n | Age | Gender (male/female) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Control | Supplementation groups | Control | Supplementation groups | Control | ||||||
| Higashikawa et al.26 | Japan | 2015 | Pediococcus pentosaceus LP28 | 1011 CFU d−1 | Adult | 21 | 20 | 52.50 ± 11.80 | 52.80 ± 11.60 | 8/13 | 7/13 |
| Rajkumar et al.25 | India | 2014 | VSL#3 | 112.5 × 109 CFU d−1 | Adult | 15 | 15 | — | — | — | — |
| Jung et al.27 | Korea | 2013 | Lactobacillus gasseri BNR17 | 6 × 1010 CFU d−1 | Adult | 28 | 29 | — | — | 13/15 | 9/20 |
| Stenman et al.28 | Finland | 2016 | Bifidobacterium animalis ssp. lactis 420(B420) | 1010 CFU d−1 | Adult | 48 | 56 | 50.60 ± 10.60 | 49.90 ± 8.50 | 9/39 | 12/44 |
| Safavi et al.29 | Iran | 2013 | Probiotic mixture① | 2.0 × 108 CFU d−1 | Children/adolescents | 29 | 27 | 10.75 ± 2.49 | 10.09 ± 1.93 | — | — |
| Ipar et al.30 | Turkey | 2015 | Probiotic mixture② | 25.4 × 108 CFU d−1 | Children/adolescents | 42 | 35 | — | — | — | — |
| Gobel et al.31 | Denmark | 2012 | L salivarius Ls-33 ATCC SD5208 | 1010 CFU d−1 | Children/adolescents | 27 | 23 | 12.90 ± 1.00 | 13.40 ± 1.10 | 11/16 | 11/12 |
| Szulińska et al.32 | Poland | 2018 | Probiotic mixture② | 1010 CFU d−1 | Women | 23 | 24 | 55.16 ± 6.87 | 58.72 ± 7.25 | 23/0 | 24/0 |
| Madjd et al.33 | UK | 2016 | Probiotic mixture④ | 107 CFU d−1 | Women | 44 | 45 | 32.20 ± 6.94 | 31.78 ± 6.81 | 44/0 | 45/0 |
| Sanchez et al.34 | Canada | 2013 | Lactobacillus rhamnosus CGMCC1.3724 (LPR) | 3.24 × 108 CFU d−1 | Adult | 45 | 48 | 35.00 ± 10.00 | 37.00 ± 10.00 | 24/38 | 24/39 |
| Ivey et al.35 | Australia | 2015 | Probiotic mixture⑤ | 3.0 × 109 CFU d−1 | Adult | 39 | 40 | 65.00 ± 7.00 | 65.00 ± 8.00 | 23/16 | 23/17 |
| Agerholm-Larsen et al.(1).36 | Denmark | 2000 | Probiotic mixture⑥ | 6 × 107 CFU d−1 | Adult | 16 | 14 | 38.60 ± 2.10 | 39.40 ± 2.10 | 12/4 | 9/5 |
| Agerholm-Larsen et al.(2).36 | Denmark | 2000 | Probiotic mixture⑦ | 18 × 108 CFU d−1 | Adult | 14 | 14 | 37.90 ± 2.40 | 39.40 ± 2.10 | 10/4 | 9/5 |
| Author | BMI | Supplementation groups | Controls | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Supplementation groups | Control | TC | LDL | HDL | TG | TC | LDL | HDL | TG | |
| ①Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium longum and Lactobacillus bulgaricus. ②Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium bifidum, Bifidobacterium longum, and Enterococcus faecium. ③Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19, and Lactococcus lactis W58. ④Streptococcus thermophiles, Lactobacillus bulgaricus, Lactobacillus acidophilus LA5 and Bifidobacterium lactis BB12. ⑤Lactobacillus acidophilus La5 and Bifidobacterium animalis subsp. lactis Bb12. ⑥Streptococcus thermophilus and Lactobacillus acidophilus. ⑦Streptococcus thermophilus and Lactobacillus rhamnosus.a Z score for the BMI. | ||||||||||
| Higashikawa et al.26 | 26.84 ± 0.25 | 27.37 ± 0.32 | −4.10 ± 5.00 | −3.60 ± 3.90 | −3.00 ± 1.20 | 65.00 ± 41.50 | −3.10 ± 4.00 | 1.70 ± 3.30 | −1.20 ± 1.10 | −3.90 ± 7.20 |
| Rajkumar et al.25 | — | — | −9.04 ± 2.85 | −8.30 ± 2.33 | 5.33 ± 0.54 | −7.57 ± 8.57 | 0.11 ± 6.82 | 0.37 ± 6.95 | −0.44 ± 1.40 | 0.85 ± 25.73 |
| Jung et al.27 | 28.60 ± 2.20 | 29.60 ± 3.60 | 5.00 ± 16.70 | 4.00 ± 20.70 | −0.70 ± 9.90 | 19.20 ± 60.80 | 1.20 ± 20.20 | 3.10 ± 18.60 | −3.90 ± 9.90 | 7.30 ± 61.80 |
| Stenman et al.28 | 31.50 ± 2.20 | 31.20 ± 2.20 | 2.71 ± 20.88 | 1.16 ± 17.79 | −1.55 ± 7.35 | 16.82 ± 46.04 | 1.93 ± 25.13 | 1.55 ± 18.56 | 0.39 ± 8.51 | 0.89 ± 35.42 |
| Safavi et al.29 | 1.79 ± 0.50a | 1.67 ± 0.39a | −3.87 ± 1.12 | −1.16 ± 0.77 | 0.39 ± 2.51 | −1.77 ± 3.81 | 0.39 ± 2.32 | 0.39 ± 0.46 | 0.00 ± 2.61 | 0.00 ± 3.45 |
| Ipar et al.30 | 27.20 ± 4.50 | 26.30 ± 3.90 | −8.40 ± 13.76 | −6.00 ± 12.77 | −2.80 ± 4.27 | −7.80 ± 24.42 | −14.10 ± 37.45 | −4.10 ± 22.58 | 2.70 ± 5.67 | −14.80 ± 28.05 |
| Gobel et al.31 | 2.60 ± 0.50a | 2.60 ± 0.40a | −8.12 ± 13.53 | −6.19 ± 11.37 | −1.16 ± 3.83 | −5.31 ± 34.09 | −3.48 ± 12.14 | −1.93 ± 10.01 | −1.16 ± 4.10 | −6.20 ± 28.33 |
| Szulińska et al.32 | 36.57 ± 5.95 | 36.10 ± 4.37 | −16.00 ± 29.24 | −4.76 ± 12.21 | 2.20 ± 7.01 | −11.64 ± 39.43 | −5.52 ± 27.52 | −2.64 ± 14.55 | 3.16 ± 7.70 | −6.04 ± 31.46 |
| Madjd et al.33 | 32.14 ± 3.20 | 32.05 ± 3.94 | −13.92 ± 11.37 | −13.92 ± 11.21 | 2.71 ± 3.71 | −15.05 ± 13.99 | −11.60 ± 11.64 | −11.60 ± 11.37 | 2.32 ± 3.33 | −15.05 ± 13.02 |
| Sanchez et al.34 | 33.80 ± 3.30 | 33.30 ± 3.20 | −7.73 ± 19.34 | −7.73 ± 15.47 | 0.00 ± 7.73 | 0.00 ± 26.56 | −3.87 ± 19.34 | −3.87 ± 15.47 | 3.87 ± 7.73 | −8.85 ± 26.56 |
| Ivey et al.35 | 31.00 ± 4.00 | 31.00 ± 4.00 | −1.93 ± 21.04 | −1.55 ± 17.05 | −0.77 ± 6.38 | 0.89 ± 34.53 | −0.39 ± 20.96 | 0.00 ± 17.98 | 0.77 ± 6.59 | −5.31 ± 33.91 |
| Agerholm-Larsen et al.(1).36 | 30.00 ± 0.70 | 30.00 ± 0.90 | 1.93 ± 4.25 | 4.64 ± 0.39 | −0.77 ± 1.16 | −0.89 ± 7.97 | 2.32 ± 6.57 | 4.25 ± 4.25 | −2.32 ± 3.48 | −0.89 ± 24.79 |
| Agerholm-Larsen et al.(2).36 | 30.20 ± 0.70 | 30.00 ± 0.90 | −1.16 ± 7.73 | −0.39 ± 7.35 | −1.55 ± 1.93 | 3.54 ± 21.25 | 2.32 ± 6.57 | 4.25 ± 4.25 | −2.32 ± 3.48 | −0.89 ± 24.79 |
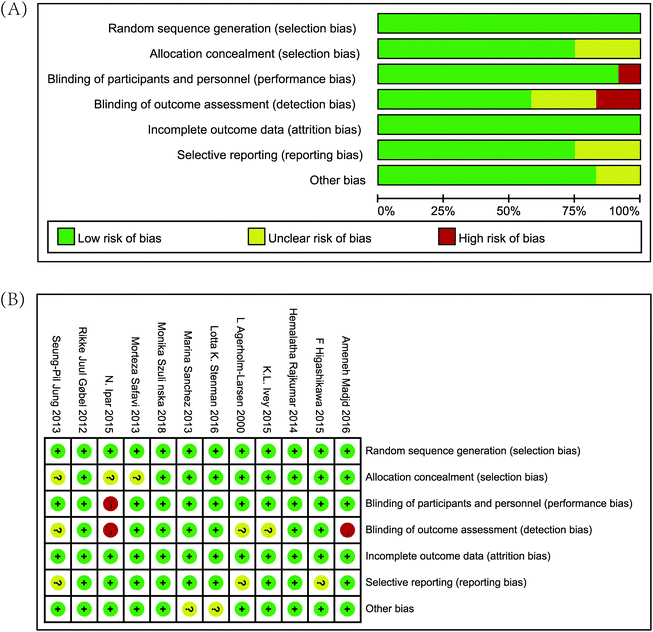
The risk of bias within individual studies is shown in Fig. 2. All 12 studies were randomized and had complete outcome data.25–36 Methods of allocation concealment were properly described in 9 studies,25,26,28,31–36 and only one study had neither a double-blind setup (for participants and study personnel) nor a blinded outcome assessment.30 Nine trials were preregistered in a clinical trial registry, which might have controlled reporting bias efficiently.25,28–35 Moreover, two studies were funded by institutions, and the institutions may have been involved;28,34 therefore, the studies were considered to have other potential bias. Simultaneously, we use the GRADE system to classify the quality of evidence for different outcomes. We are confident that the true effect lies close to that of the estimate of the effect (Table 2).
| Probiotic supplementation compared to no probiotic intervention for regulating blood lipid levels |
| Population: Overweight or obese subjects |
| Settings: Five studies were conducted in Asia, five studies were conducted in Europe, and the other studies were conducted in Oceania and America |
| Intervention: Probiotic supplementation |
| Comparison: No probiotic intervention |
| Outcomea | WMD (95% CI)b | No. of participants (studies) | Quality of the evidence comments (GRADE) |
|---|---|---|---|
| TC level | −3.04 (−4.88, −1.21) mgdL−1 | 767 (12RCTs) | ⊕⊕⊕⊕High |
| LDL level | −2.28 (−3.60, −0.96) mg dL−1 | 737 (11RCTs) | ⊕⊕⊕⊕High |
| HDL level | −0.26 (−2.39, 1.87) mg dL−1 | 767 (12RCTs) | ⊕⊕⊕○Moderatec |
| TG level | −0.86 (−2.54, 0.83) mg dL−1 | 726 (11RCTs) | ⊕⊕⊕⊕High |
| a All subjects were followed up, ranging from 6 weeks to 6 months. b Results for the variations in the treatments compared with the controls. c Downgraded by one level due to high heterogeneity. WMD: weight mean deviation; CI: confidence interval; RCT: randomized controlled trial. |
|---|
| GRADE working group grades of evidence |
| High quality: We are very confident that the true effect lies close to that of the estimate of the effect |
| Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect |
| Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect |
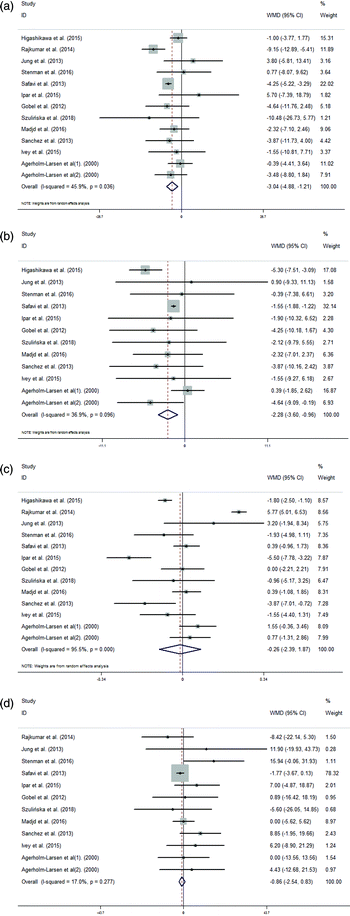
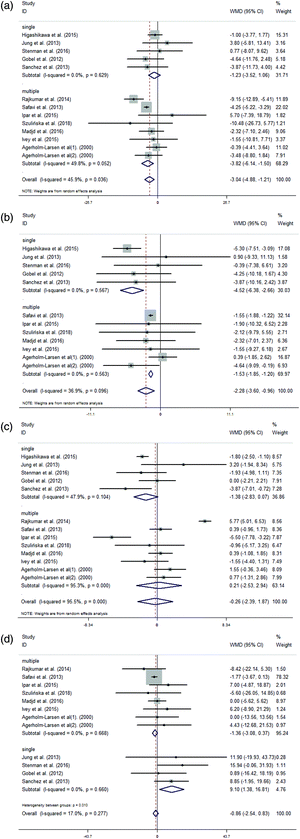
We conducted a meta-analysis of the TC, LDL, HDL and TG concentrations in 391 treatment and 376 control subjects. The group that was administered probiotics was associated with a significantly larger reduction in TC levels (WMD = −3.04 mg dL−1, 95% CI = −4.88, −1.21 mg dL−1, I2 = 45.9%, p = 0.036; Fig. 3(A)) compared with the control group. Statistically significant differences in the variations in LDL concentrations were observed between the supplementation group and the control group (WMD = −2.28 mg dL−1, 95% CI = −3.60, −0.96 mg dL−1, I2 = 36.9%, p = 0.096; Fig. 3(B)). However, there were no statistically significant differences in the variations in HDL concentrations between the supplementation group and the control group (WMD = −0.26 mg dL−1, 95% CI = −2.39, 1.87 mg dL−1, I2 = 95.5%, p < 0.001; Fig. 3(C)). Additionally, an analysis of the results of these studies indicated that there were no significant differences between the supplementation group and the control group in terms of the variations in TG concentrations (WMD = −0.86 mg dL−1, 95% CI = −2.54, 0.83 mg dL−1, I2 = 17.0%, p = 0.277; Fig. 3(D)).
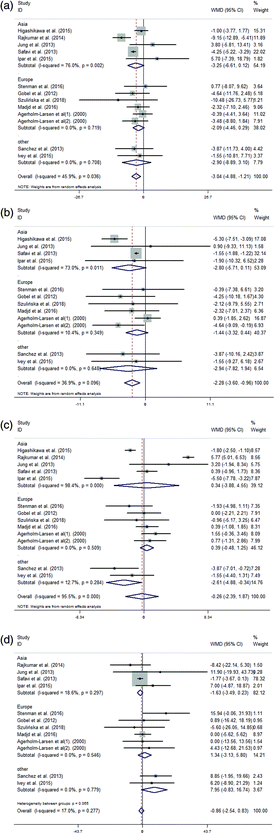
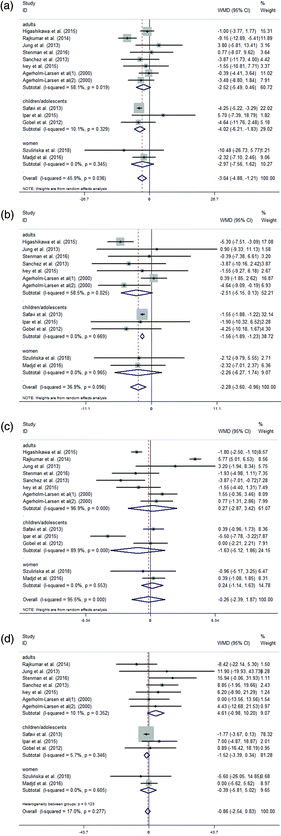
Subgroup analysis was performed according to the object, region and probiotics intervention. Some details are presented in Table 3. Studies were divided into three areas: Asia, Europe and others (America and Oceania) according to the geographical study area. The studies in all three areas showed that the TC, LDL and TG variations in the supplementation group were not significantly different from those in the control group (Fig. 4(A), (B), and (D)). Additionally, in the subgroup meta-analysis of the variations in HDL concentrations, the studies in Asia and Europe did not demonstrate differences between the probiotic and control groups (Fig. 4(C)).
| Number of studies | Weighted mean difference (95% CI) | Heterogeneity (I2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | LDL | HDL | TG | TC | LDL | HDL | TG | TC | LDL | HDL | TG | |
| L: Lactobacillus. L + S: Lactobacillus + Streptococcus. L + S + B: Lactobacillus + Streptococcus + Bifidobacterium. | ||||||||||||
| Region | ||||||||||||
| Asia | 5 | 4 | 5 | 4 | −3.25 (−6.61, 0.12) | −2.80 (−5.71, 0.11) | 0.34 (−3.88, 4.55) | −1.63 (−3.49, 0.23) | 76.0% | 73.0% | 98.4% | 18.6% |
| Europe | 5 | 5 | 5 | 5 | −2.09 (−4.46, 0.29) | −1.44 (−3.32, 0.44) | 0.39 (−0.48, 1.25) | 1.34 (−3.13, 5.80) | 0.0% | 10.4% | 0.0% | 0.0% |
| Other areas | 2 | 2 | 2 | 2 | −2.90 (−8.89, 3.10) | −2.94 (−7.82, 1.94) | −2.61 (−4.88, −0.34) | 7.95 (−0.83, 16.74) | 0.0% | 0.0% | 12.7% | 0.0% |
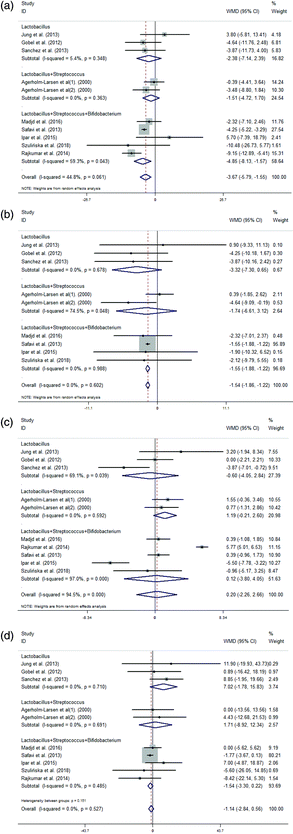
| Number of probiotics | ||||||||||||
| Single | 5 | 5 | 5 | 4 | −1.23 (−3.52, 1.06) | −4.52 (−6.38, −2.66) | −1.38 (−2.83, 0.07) | 9.10 (1.38, 16.81) | 0.0% | 0.0% | 47.9% | 0.0% |
| Multiple | 7 | 6 | 7 | 7 | −3.82 (−6.14, −1.50) | −1.53 (−1.85, −1.20) | 0.21 (−2.53, 2.94) | −1.36 (−3.08, 0.37) | 49.8% | 0.0% | 95.3% | 0.0% |
| Probiotic species | ||||||||||||
| L | 3 | 3 | 3 | 3 | −2.38 (−7.14, 2.39) | −3.32 (−7.30, 0.65) | −0.60 (−4.05, 2.84) | 7.02 (−1.78, 15.83) | 5.4% | 0.0% | 69.1% | 0.0% |
| L + S | 1 | 1 | 1 | 1 | −1.51 (−4.72, 1.70) | −1.74 (−6.61, 3.12) | 1.19 (−0.21, 2.60) | 1.71 (−8.92, 12.34) | 0.0% | 74.5% | 0.0% | 0.0% |
| L + S + B | 5 | 4 | 5 | 5 | −4.85 (−8.13,−1.57) | −1.55 (−1.88,−1.22) | 0.12 (−3.80, 4.05) | −1.54 (−3.30, 0.22) | 59.3% | 0.0% | 97.0% | 0.0% |
| Subjects | ||||||||||||
| Adults | 7 | 6 | 7 | 6 | −2.52 (−5.49, 0.46) | −2.51 (−5.15, 0.13) | 0.27 (−2.87, 3.42) | 4.61 (−0.98, 10.20) | 58.1% | 58.5% | 96.9% | 10.1% |
| Children/adolescents | 3 | 3 | 3 | 3 | −4.02 (−6.21, −1.83) | −1.56 (−1.89, −1.23) | −1.63 (−5.12, 1.86) | −1.52 (−3.39, 0.34) | 10.1% | 0.0% | 89.9% | 5.7% |
| Women | 2 | 2 | 2 | 2 | −2.97 (−7.56, 1.62) | −2.26 (−6.27, 1.74) | 0.24 (−1.14, 1.63) | −0.39 (−5.81, 5.02) | 0.0% | 0.0% | 0.0% | 0.0% |
The included articles were divided into two groups according to the probiotics as follows: single probiotic group and multiple probiotic group. In the multiple probiotic group, the intervention group showed a larger reduction in the variations in TC concentrations than the control group (Fig. 5(A)). The meta-analysis that included the variations in LDL concentrations showed that the administration of probiotics was associated with a significantly larger reduction in both the single probiotic group and the multiple probiotic group compared with that in the control group (Fig. 5(B)). The subgroup analysis that included the variations in HDL concentrations showed an overall nonsignificant effect between the single probiotic group and the multiple probiotic group (Fig. 5(C)). Moreover, the intervention group showed an increment in the variations in TG concentrations compared with the control group in the single probiotic group (Fig. 5(D)).
We divided the studies into three subgroups (adults, children/adolescents, and women) according to the subjects. The administration of probiotics was associated with a significantly larger reduction of TC and LDL concentrations in children/adolescents compared with that in the control group (Fig. 6(A) and (B)). Additionally, the subgroup analysis showed that there were no significant differences among adults, children/adolescents, and women between the probiotic and control groups in terms of the variations in HDL and TG concentrations (Fig. 6(C) and (D)).
The included articles were divided into three groups according to the probiotic species as follows: L, L + S and L + S + B (other probiotic species groups only had one study, so we could not analyse them via subgroup analysis). The intervention group showed a larger reduction in the variations in TC and LDL concentrations than the control group in the Lactobacillus + Streptococcus + Bifidobacterium group (Fig. 7(A) and (B)). However, the subgroup analysis showed that there were no significant differences among the three groups between the probiotic and control groups in terms of the variations in HDL and TG concentrations (Fig. 7(C) and (D)).
4. Discussion
Abnormal levels of blood lipids, particularly higher concentrations of TC and LDL, are major determining factors for cardiovascular disease. Additionally, the LDL or TC levels have an independent predictive effect on the risk of arteriosclerotic cardiovascular disease (ASCVD).15,37 It has been reported that probiotic supplementation could reduce body weight, BMI and fat percentage.38 However, it is unclear whether supplementation with probiotics is required or indeed beneficial to reduce blood lipid levels in obese or overweight people. This meta-analysis found that obese or overweight participants receiving probiotic supplementation had significantly larger reductions in TC and LDL concentrations than the control subjects. Although the specific mechanism of the effect of probiotics on TC and LDL has not yet been elucidated, several possible mechanisms have been proposed. Probiotics can bind cholesterol to reduce cholesterol absorption in the intestine.39 Additionally, probiotics could reduce the enterohepatic circulation of bile salts, prompting the liver to mobilize more cholesterol to re-synthesize bile salts, thus reducing the cholesterol levels.40–42 The meta-analysis implies a stronger effect of probiotics on TC and LDL than on the TG and HDL levels, and it has been suggested that probiotics can promote the excretion of cholesterol and bile acid via the alteration of the pathways of cholesterol esters and lipoprotein transporters, without affecting the synthesis of hepatic cholesterol.41,43 Therefore, the administration of probiotics may reduce the risk of ASCVD and other cardiovascular diseases in obese or overweight people.According to the results of the subgroup meta-analysis of the variations in TC and LDL concentrations, the administration of probiotics was associated with a significantly larger reduction in their concentration in children/adolescents compared with that in the controls. At present, intestinal microflora are known to regulate blood lipid levels in two different ways. One is through the regulation of bile acid metabolism, thus affecting subsequent metabolic processes and leading to changes in blood lipid levels.44 The other one is the production of SCFAs from indigestible polysaccharides. SCFAs such as acetate, butyrate and propionate are produced by the bacterial fermentation function as energy substrates, as well as regulators of satiety and food intake. SCFAs are also involved in the regulation of energy metabolism and insulin sensitivity in peripheral tissues by the activation of the G-protein-coupled receptors GPR41 and GPR43 on the intestinal epithelial cells.45 The understanding of intestinal microflora in children is in its infancy; multiple endogenous and exogenous factors can influence the intestinal microflora in children, thus influencing blood lipid levels in children.46 Probiotics are a mainstay of treatment directed at the modification of the intestinal microflora.47 Therefore, probiotic supplementation may be more conducive to the recovery of the intestinal microflora balance in children and thus may play a role in the regulation of blood lipid levels in children.
According to the results of the meta-analysis, probiotic supplementation in obese or overweight people was associated with a significantly larger reduction in TC and LDL concentrations compared to that in the control subjects. However, when a subgroup analysis was performed according to the subjects of the studies, there were no significant differences between the probiotic and control groups in women. This result may be due to the small sample size and limited studies.
We found that the multiple probiotic groups showed a larger reduction in the variations in TC concentrations compared with the control group. However, the variations in the TC concentrations in the single probiotic group were not significantly different from those in the control group. Previous studies have found that multiple strain probiotics appear to be more feasible and effective than single strain probiotics for the prevention of necrotizing enterocolitis (NEC) and the reduction of mortality in preterm very low birth weight (PVLBW) neonates.48 Our study also indicated that the L + S + B group showed a larger reduction in the variations in TC and LDL concentrations compared with the control group. In some findings, the intervention of lactobacilli and bifidobacteria was negatively correlated with obesity and could provide anti-obesity effects.49–52 What's more, studies have proved that VSL#3, a freeze-dried pharmaceutical probiotic preparation containing all three strains of Lactobacillus, Bifidobacterium and Streptococcus, could attenuate obesity and diabetes via the modulation of the intestinal microflora, as well as the improvement of NAFLD.53,54 Therefore, we considered that in the regulation of blood lipid levels, multiple strain probiotics appear to be more effective than single strain probiotics. Simultaneously, there was an increment in the variations in the TG concentrations in the single probiotic group. This result could be due to the small sample size and limited studies because wider confidence intervals were observed. Consequently, follow-up clinical trials and a supporting mechanistic theory are still needed.
According to the results of the meta-analysis, a large degree of heterogeneity was observed for the variations in HDL concentrations. We therefore performed an analysis to identify the source of heterogeneity, and the heterogeneity was lower in the European group when grouped by geographical study area. Therefore, we considered that different geographical study areas may be the sources of heterogeneity in the associated studies. A large degree of heterogeneity was observed in the Asian group, which may be a result of the thousands of ethnic groups in Asia, accounting for approximately 80% of the world's total. The physiological and biochemical levels among different Asian races may be different. This hypothesis requires additional data from large clinical trials on Asian populations.
This study has some limitations. A few studies included more than one probiotic intervention, and we chose to include the intervention groups that received the highest daily dose over the longest time period, as we considered these groups more likely to experience an effect on the blood lipid levels and related outcomes. This meta-analysis included only quantitative results with specific values, and studies with qualitative results were not included. Most importantly, there were fewer studies from Asia, possibly because there are more races in Asia and large differences between races, and we found that the heterogeneity was higher in the Asian group when a subgroup analysis was conducted according to the geographical study area. The effect of probiotics on the variations in HDL concentrations in this meta-analysis should be interpreted with caution. Because this is a meta-analysis of randomized controlled trials, the quality of this study is affected by the quality of data from the original publications. Although randomized trials are considered valid compared to other studies, true intervention effects could be biased by limited methodological quality.15
5. Conclusion
In summary, this meta-analysis found that probiotic supplementation could improve lipid metabolism, particularly by reducing TC and LDL concentrations in obese or overweight people. Considering several limitations observed in this meta-analysis and previous clinical trials, additional data from large clinical trials are required to confirm the efficacy and safety of probiotics for the regulation of blood lipid levels in obese or overweight people.Author contributions
BL, WC and SY designed the study; SY, ZT and ML performed the study; SY and ZT analyzed the data and drafted the manuscript; SY and ML participated in amending the manuscript. All authors approved the final version of the manuscript.Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81773412).References
- D. K. Dahiya, Renuka, M. Puniya, U. K. Shandilya, T. Dhewa, N. Kumar, S. Kumar, A. K. Puniya and P. Shukla, Gut Microbiota Modulation and Its Relationship with Obesity Using Prebiotic Fibers and Probiotics: A Review, Front. Microbiol., 2017, 8, 563 CrossRef PubMed.
- P. Kopelman, Health risks associated with overweight and obesity, Obesity Rev., 2007, 8(Suppl 1), 13–17 CrossRef PubMed.
- A. Nikolopoulou and N. P. Kadoglou, Obesity and metabolic syndrome as related to cardiovascular disease, Expert Rev. Cardiovasc. Ther., 2012, 10, 933–939 CrossRef CAS PubMed.
- I. Vucenik and J. P. Stains, Obesity and cancer risk: evidence, mechanisms, and recommendations, Ann. N. Y. Acad. Sci., 2012, 1271, 37–43 CrossRef CAS PubMed.
- A. Afshin, M. H. Forouzanfar, M. B. Reitsma, P. Sur, K. Estep, A. Lee, L. Marczak, A. H. Mokdad, M. Moradi-Lakeh, M. Naghavi, J. S. Salama, T. Vos, K. H. Abate, C. Abbafati, M. B. Ahmed, Z. Al-Aly, A. Alkerwi, R. Al-Raddadi, A. T. Amare, A. Amberbir, A. K. Amegah, E. Amini, S. M. Amrock, R. M. Anjana, J. Arnlov, H. Asayesh, A. Banerjee, A. Barac, E. Baye, D. A. Bennett, A. S. Beyene, S. Biadgilign, S. Biryukov, E. Bjertness, D. J. Boneya, I. Campos-Nonato, J. J. Carrero, P. Cecilio, K. Cercy, L. G. Ciobanu, L. Cornaby, S. A. Damtew, L. Dandona, R. Dandona, S. D. Dharmaratne, B. B. Duncan, B. Eshrati, A. Esteghamati, V. L. Feigin, J. C. Fernandes, T. Furst, T. T. Gebrehiwot, A. Gold, P. N. Gona, A. Goto, T. D. Habtewold, K. T. Hadush, N. Hafezi-Nejad, S. I. Hay, M. Horino, F. Islami, R. Kamal, A. Kasaeian, S. V. Katikireddi, A. P. Kengne, C. N. Kesavachandran, Y. S. Khader, Y. H. Khang, J. Khubchandani, D. Kim, Y. J. Kim, Y. Kinfu, S. Kosen, T. Ku, B. K. Defo, G. A. Kumar, H. J. Larson, M. Leinsalu, X. Liang, S. S. Lim, P. Liu, A. D. Lopez, R. Lozano, A. Majeed, R. Malekzadeh, D. C. Malta, M. Mazidi, C. McAlinden, S. T. McGarvey, D. T. Mengistu, G. A. Mensah, G. B. M. Mensink, H. B. Mezgebe, E. M. Mirrakhimov, U. O. Mueller, J. J. Noubiap, C. M. Obermeyer, F. A. Ogbo, M. O. Owolabi, G. C. Patton, F. Pourmalek, M. Qorbani, A. Rafay, R. K. Rai, C. L. Ranabhat, N. Reinig, S. Safiri, J. A. Salomon, J. R. Sanabria, I. S. Santos, B. Sartorius, M. Sawhney, J. Schmidhuber, A. E. Schutte, M. I. Schmidt, S. G. Sepanlou, M. Shamsizadeh, S. Sheikhbahaei, M. J. Shin, R. Shiri, I. Shiue, H. S. Roba, D. A. S. Silva, J. I. Silverberg, J. A. Singh, S. Stranges, S. Swaminathan, R. Tabares-Seisdedos, F. Tadese, B. A. Tedla, B. S. Tegegne, A. S. Terkawi, J. S. Thakur, M. Tonelli, R. Topor-Madry, S. Tyrovolas, K. N. Ukwaja, O. A. Uthman, M. Vaezghasemi, T. Vasankari, V. V. Vlassov, S. E. Vollset, E. Weiderpass, A. Werdecker, J. Wesana, R. Westerman, Y. Yano, N. Yonemoto, G. Yonga, Z. Zaidi, Z. M. Zenebe, B. Zipkin and C. J. L. Murray, Health Effects of Overweight and Obesity in 195 Countries over 25 Years, N. Engl. J. Med., 2017, 377, 13–27 CrossRef PubMed.
- FAO, Guidelines for the evaluation of probiotics in food, 2002 Search PubMed.
- V. Theodorou, A. A. Belgnaoui, S. Agostini and H. Eutamene, Effect of commensals and probiotics on visceral sensitivity and pain in irritable bowel syndrome, Gut Microbes, 2014, 5, 430–629 CrossRef PubMed.
- K. Dylag, M. Hubalewska-Mazgaj, M. Surmiak, J. Szmyd and T. Brzozowski, Probiotics in the mechanism of protection against gut inflammation and therapy of gastrointestinal disorders, Curr. Pharm. Des., 2014, 20, 1149–1155 CrossRef CAS.
- M. Roberfroid, G. R. Gibson, L. Hoyles, A. L. McCartney, R. Rastall, I. Rowland, D. Wolvers, B. Watzl, H. Szajewska, B. Stahl, F. Guarner, F. Respondek, K. Whelan, V. Coxam, M. J. Davicco, L. Leotoing, Y. Wittrant, N. M. Delzenne, P. D. Cani, A. M. Neyrinck and A. Meheust, Prebiotic effects: metabolic and health benefits, Br. J. Nutr., 2010, 104(Suppl 2), S1–63 CrossRef CAS PubMed.
- V. W. Wong, G. L. Won, A. M. Chim, W. C. Chu, D. K. Yeung, K. C. Li and H. L. Chan, Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study, Ann. Hepatol., 2013, 12, 256–262 CAS.
- A. Sepideh, P. Karim, A. Hossein, R. Leila, M. Hamdollah, E. G. Mohammad, S. Mojtaba, S. Mohammad, G. Ghader and A. Seyed Moayed, Effects of Multistrain Probiotic Supplementation on Glycemic and Inflammatory Indices in Patients with Nonalcoholic Fatty Liver Disease: A Double-Blind Randomized Clinical Trial, J. Am. Coll. Nutr., 2016, 35, 500–505 CrossRef CAS PubMed.
- A. Lee, Y. J. Lee, H. J. Yoo, M. Kim, Y. Chang, D. S. Lee and J. H. Lee, Consumption of Dairy Yogurt Containing Lactobacillus paracasei ssp. paracasei, Bifidobacterium animalis ssp. lactis and Heat-Treated Lactobacillus plantarum Improves Immune Function Including Natural Killer Cell Activity, Nutrients, 2017, 9, 558 CrossRef PubMed.
- P. Q. Dos Santos, J. C. Guedes, R. P. de Jesus, R. R. D. Santos and R. L. Fiaconne, Effects of using symbiotics in the clinical nutritional evolution of patients with chronic pancreatitis: Study prospective, randomized, controlled, double blind, Clin. Nutr. ESPEN, 2017, 18, 9–15 CrossRef PubMed.
- M. Uccello, G. Malaguarnera, F. Basile, V. D'Agata, M. Malaguarnera, G. Bertino, M. Vacante, F. Drago and A. Biondi, Potential role of probiotics on colorectal cancer prevention, BMC Surg., 2012, 12(Suppl 1), S35 CrossRef.
- Y. A. Cho and J. Kim, Effect of Probiotics on Blood Lipid Concentrations: A Meta-Analysis of Randomized Controlled Trials, Medicine, 2015, 94, e1714 CrossRef PubMed.
- A. Riva, F. Borgo, C. Lassandro, E. Verduci, G. Morace, E. Borghi and D. Berry, Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations, Dig. Liver Dis., 2017, 19, 95–105 CAS.
- Y. T. Tsai, P. C. Cheng and T. M. Pan, Anti-obesity effects of gut microbiota are associated with lactic acid bacteria, Appl. Microbiol. Biotechnol., 2014, 98, 1–10 CrossRef CAS PubMed.
- B. Richelsen, K. Kristensen and S. B. Pedersen, Long-term (6 months) effect of a new fermented milk product on the level of plasma lipoproteins–a placebo-controlled and double blind study, Eur. J. Clin. Nutr., 1996, 50, 811–815 CAS.
- J. Z. Xiao, S. Kondo, N. Takahashi, K. Miyaji, K. Oshida, A. Hiramatsu, K. Iwatsuki, S. Kokubo and A. Hosono, Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteers, J. Dairy Sci., 2003, 86, 2452–2461 CrossRef CAS PubMed.
- G. Kiessling, J. Schneider and G. Jahreis, Long-term consumption of fermented dairy products over 6 months increases HDL cholesterol, Eur. J. Clin. Nutr., 2002, 56, 843–849 CrossRef CAS PubMed.
- J. P. Higgins, D. G. Altman, P. C. Gotzsche, P. Juni, D. Moher, A. D. Oxman, J. Savovic, K. F. Schulz, L. Weeks and J. A. Sterne, The Cochrane Collaboration's tool for assessing risk of bias in randomised trials, BMJ, 2011, 343, d5928 CrossRef PubMed.
- G. S . Higgins JPT, Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011], 2011 Search PubMed.
- W. G. Cochran, The combination of estimates from different experiments, Biometrics, 1954, 10, 101–129 CrossRef.
- J. P. Higgins, S. G. Thompson, J. J. Deeks and D. G. Altman, Measuring inconsistency in meta-analyses, BMJ, 2003, 327, 557–560 CrossRef PubMed.
- H. Rajkumar, N. Mahmood, M. Kumar, S. R. Varikuti, H. R. Challa and S. P. Myakala, Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial, Mediators Inflammation, 2014, 2014, 348959 Search PubMed.
- F. Higashikawa, M. Noda, T. Awaya, N. Danshiitsoodol, Y. Matoba, T. Kumagai and M. Sugiyama, Antiobesity effect of Pediococcus pentosaceus LP28 on overweight subjects: a randomized, double-blind, placebo-controlled clinical trial, Eur. J. Clin. Nutr., 2016, 70, 582–587 CrossRef CAS PubMed.
- S. P. Jung, K. M. Lee, J. H. Kang, S. I. Yun, H. O. Park, Y. Moon and J. Y. Kim, Effect of Lactobacillus gasseri BNR17 on Overweight and Obese Adults: A Randomized, Double-Blind Clinical Trial, Korean J. Fam. Med., 2013, 34, 80–89 CrossRef PubMed.
- L. K. Stenman, M. J. Lehtinen, N. Meland, J. E. Christensen, N. Yeung, M. T. Saarinen, M. Courtney, R. Burcelin, M. L. Lahdeaho, J. Linros, D. Apter, M. Scheinin, H. Kloster Smerud, A. Rissanen and S. Lahtinen, Probiotic With or Without Fiber Controls Body Fat Mass, Associated With Serum Zonulin, in Overweight and Obese Adults-Randomized Controlled Trial, EBioMedicine, 2016, 13, 190–200 CrossRef PubMed.
- M. Safavi, S. Farajian, R. Kelishadi, M. Mirlohi and M. Hashemipour, The effects of synbiotic supplementation on some cardio-metabolic risk factors in overweight and obese children: a randomized triple-masked controlled trial, Int. J. Food Sci. Nutr., 2013, 64, 687–693 CrossRef CAS PubMed.
- N. Ipar, S. D. Aydogdu, G. K. Yildirim, M. Inal, I. Gies, Y. Vandenplas and E. C. Dinleyici, Effects of synbiotic on anthropometry, lipid profile and oxidative stress in obese children, Benefic. Microbes, 2015, 6, 775–782 CrossRef CAS PubMed.
- R. J. Gobel, N. Larsen, M. Jakobsen, C. Molgaard and K. F. Michaelsen, Probiotics to adolescents with obesity: effects on inflammation and metabolic syndrome, J. Pediatr. Gastroenterol. Nutr., 2012, 55, 673–678 CrossRef PubMed.
- M. Szulińska, I. Łoniewski, S. V. Hemert, M. Sobieska and P. Bogdański, Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial, Nutrients, 2018, 10, 773 CrossRef PubMed.
- A. Madjd, M. A. Taylor, N. Mousavi, A. Delavari, R. Malekzadeh, I. A. Macdonald and H. R. Farshchi, Comparison of the effect of daily consumption of probiotic compared with low-fat conventional yogurt on weight loss in healthy obese women following an energy-restricted diet: a randomized controlled trial, Am. J. Clin. Nutr., 2016, 103, 323–329 CrossRef CAS PubMed.
- M. Sanchez, C. Darimont, V. Drapeau, S. Emady-Azar, M. Lepage, E. Rezzonico, C. Ngom-Bru, B. Berger, L. Philippe, C. Ammon-Zuffrey, P. Leone, G. Chevrier, E. St-Amand, A. Marette, J. Dore and A. Tremblay, Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women, Br. J. Nutr., 2014, 111, 1507–1519 CrossRef CAS PubMed.
- K. L. Ivey, J. M. Hodgson, D. A. Kerr, P. L. Thompson, B. Stojceski and R. L. Prince, The effect of yoghurt and its probiotics on blood pressure and serum lipid profile; a randomised controlled trial, Nutr., Metab., Cardiovas. Dis., 2015, 25, 46–51 CrossRef CAS PubMed.
- L. Agerholm-Larsen, A. Raben, N. Haulrik, A. S. Hansen, M. Manders and A. Astrup, Effect of 8 weeks intake of probiotic milk products on risk factors for cardiovascular diseases, Eur. J. Clin. Nutr., 2000, 54, 288–297 CrossRef CAS PubMed.
- D. Zhao, J. Liu, W. Xie and Y. Qi, Cardiovascular risk assessment: a global perspective, Nat. Rev. Cardiol., 2015, 12, 301–311 CrossRef PubMed.
- H. Borgeraas, L. K. Johnson, J. Skattebu, J. K. Hertel and J. Hjelmesaeth, Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials, Obesity Rev., 2018, 19, 219–232 CrossRef CAS PubMed.
- S. E. Gilliland, C. R. Nelson and C. Maxwell, Assimilation of cholesterol by Lactobacillus acidophilus, Appl. Environ. Microbiol., 1985, 49, 377–381 CAS.
- G. B. Kim, S. H. Yi and B. H. Lee, Purification and Characterization of Three Different Types of Bile Salt Hydrolases from Bifidobacterium Strains, J. Dairy Sci., 2004, 87, 258–266 CrossRef CAS PubMed.
- L. Min-Tze, F. R. Dunshea and N. P. Shah, Effects of a synbiotic containing Lactobacillus acidophilus ATCC 4962 on plasma lipid profiles and morphology of erythrocytes in hypercholesterolaemic pigs on high- and low-fat diets, Br. J. Nutr., 2007, 98, 736–744 Search PubMed.
- I. De Smet, P. De Boever and W. Verstraete, Cholesterol lowering in pigs through enhanced bacterial bile salt hydrolase activity, Br. J. Nutr., 1998, 79, 185–194 CrossRef CAS PubMed.
- J. Z. Xiao, S. Kondo, N. Takahashi, K. Miyaji, K. Oshida, A. Hiramatsu, K. Iwatsuki, S. Kokubo and A. Hosono, Effects of Milk Products Fermented by Bifidobacterium longum on Blood Lipids in Rats and Healthy Adult Male Volunteers, J. Dairy Sci., 2003, 86, 2452–2461 CrossRef CAS PubMed.
- J. Fu, M. J. Bonder, M. C. Cenit, E. F. Tigchelaar, A. Maatman, J. A. Dekens, E. Brandsma, J. Marczynska, F. Imhann, R. K. Weersma, L. Franke, T. W. Poon, R. J. Xavier, D. Gevers, M. H. Hofker, C. Wijmenga and A. Zhernakova, The Gut Microbiome Contributes to a Substantial Proportion of the Variation in Blood Lipids, Circ. Res., 2015, 117, 817–824 CrossRef CAS PubMed.
- A. J. Brown, S. M. Goldsworthy, A. A. Barnes, M. M. Eilert, L. Tcheang, D. Daniels, A. I. Muir, M. J. Wigglesworth, I. Kinghorn, N. J. Fraser, N. B. Pike, J. C. Strum, K. M. Steplewski, P. R. Murdock, J. C. Holder, F. H. Marshall, P. G. Szekeres, S. Wilson, D. M. Ignar, S. M. Foord, A. Wise and S. J. Dowell, The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids, J. Biol. Chem., 2003, 278, 11312–11319 CrossRef CAS PubMed.
- J. Bai, M. Behera and D. W. Bruner, The gut microbiome, symptoms, and targeted interventions in children with cancer: a systematic review, Support. Care Cancer, 2018, 26, 427–439 CrossRef PubMed.
- N. Kobyliak, C. Conte, G. Cammarota, A. P. Haley, I. Styriak, L. Gaspar, J. Fusek, L. Rodrigo and P. Kruzliak, Probiotics in prevention and treatment of obesity: a critical view, Nutr. Metab., 2016, 13, 14 CrossRef.
- H. Y. Chang, J. H. Chen, J. H. Chang, H. C. Lin, C. Y. Lin and C. C. Peng, Multiple strains probiotics appear to be the most effective probiotics in the prevention of necrotizing enterocolitis and mortality: An updated meta-analysis, PLoS One, 2017, 12, e0171579 CrossRef PubMed.
- F. Armougom, M. Henry, B. Vialettes, D. Raccah and D. Raoult, Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients, PLoS One, 2009, 4, e7125 CrossRef PubMed.
- M. Million, M. Maraninchi, M. Henry, F. Armougom, H. Richet, P. Carrieri, R. Valero, D. Raccah, B. Vialettes and D. Raoult, Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii, Int. J. Obes., 2012, 36, 817–825 CrossRef CAS PubMed.
- Y. N. Yin, Q. F. Yu, N. Fu, X. W. Liu and F. G. Lu, Effects of four Bifidobacteria on obesity in high-fat diet induced rats, World J. Gastroenterol., 2010, 16, 3394 CrossRef CAS PubMed.
- H. M. An, S. Y. Park, D. K. Lee, J. R. Kim, M. K. Cha, S. W. Lee, H. T. Lim, K. J. Kim and N. J. Ha, Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats, Lipids Health Dis., 2011, 10, 116 CrossRef PubMed.
- Y. Hariom, L. Ji-Hyeon, L. John, W. Peter and S. G. Rane, Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion, J. Biol. Chem., 2013, 288, 25088–25097 CrossRef PubMed.
- A. Alisi, G. Bedogni, G. Baviera, V. Giorgio, E. Porro, C. Paris, P. Giammaria, L. Reali, F. Anania and V. Nobili, Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis, Aliment. Pharmacol. Ther., 2014, 39, 1276–1285 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8fo02163e |
| This journal is © The Royal Society of Chemistry 2019 |