Towards the development of a standardized method for extraction and analysis of PFAS in biological tissues†
Abstract
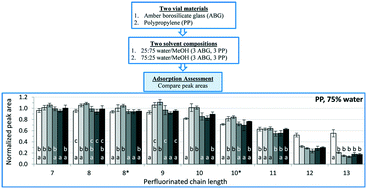
The stability and surface activity of per- and polyfluoroalkyl substances (PFAS) led to their extensive and diverse commercial and industrial use. However, these properties are accompanied by generally high aqueous solubility and toxic effects, resulting in growing scientific and public concern. Given the surge in PFAS analyses in recent years, recurrent incidents of analytical inconsistencies reported in the literature, and the demand for robust, standardized analytical methods, an effort to condense and quantitatively define/describe PFAS analytical issues was necessary. Thus, this work had two objectives. A review of PFAS analytical literature identified and compiled analytical challenges with specific attention to interlaboratory studies and information related to low analyte recoveries. Data from a series of systematic experiments quantitatively helped to identify those differences in the analytical methodologies that caused inconsistencies and may compromise PFAS analytical results. The adsorptive properties of these unique compounds were identified as the primary source of analytical irreproducibility, particularly for analytes with a perfluoroalkyl chain length exceeding nine carbons. More specifically, solvent composition (water/methanol ratio) was found to be the primary contributor to adsorptive analyte losses with a significant increase in adsorption occurring when water content of the analyzed solution was raised beyond 25%. The container material used also affected adsorptive losses for solutions with elevated water fractions. Contrary to literature reports, polypropylene containers generally resulted in greater analyte losses compared to amber borosilicate glass containers. These findings led to modifications of sample preparation procedures to provide a more robust analytical methodology. This methodology was subsequently utilized in the analysis of 300 biological tissue samples. A detailed procedure and discussion of its performance are provided. These findings and the connections made to the cited literature will provide the framework for a standardized method for PFAS extraction and analysis of biological tissues.
- This article is part of the themed collection: PFAS


 Please wait while we load your content...
Please wait while we load your content...