Open Access Article
Open Access ArticleTemperature-dependent mechanisms of DOM removal by biological activated carbon filters
Nashita
Moona
 *a,
Urban J.
Wünsch
*a,
Urban J.
Wünsch
 a,
Mia
Bondelind
a,
Mia
Bondelind
 a,
Olof
Bergstedt
a,
Olof
Bergstedt
 b,
Tugba
Sapmaz
b,
Tugba
Sapmaz
 c,
Thomas J. R.
Pettersson
c,
Thomas J. R.
Pettersson
 a and
Kathleen R.
Murphy
a and
Kathleen R.
Murphy
 *a
*a
aArchitecture and Civil Engineering, Water Environment Technology, Chalmers University of Technology, Sven Hultins Gata 6, 41296 Gothenburg, Sweden. E-mail: n_moona@hotmail.com; murphyk@chalmers.se
bGöteborgs Stad Kretslopp och vatten, Gothenburg, Sweden
cDepartment of Environmental Engineering, Istanbul Technical University, Maslak, 34469 Istanbul, Turkey
First published on 14th October 2019
Abstract
Seasonal variability in the removal of dissolved organic matter (DOM) by drinking water biological activated carbon (BAC) filters is often attributed to temperature changes. However, it can be rather difficult to directly relate temperature to treatment efficiency at full scale due to seasonal variations in other influential parameters like DOM concentration and character, and microbial activity. Furthermore, processes in BAC filters include adsorption, desorption and biodegradation within biofilms while each respond differently to temperature. This study aimed to decouple these processes by studying the removal of various DOM fractions from coagulated and settled drinking water when in contact with aged (>3 years) BAC filter material at different water temperatures. DOM removal was measured as changes in dissolved organic carbon (DOC), ultraviolet absorbance at 254 nm (UV254) and fluorescence. Under the particular experimental conditions there was little evidence of biological removal; instead, removal of DOM fractions emitting at longer wavelengths (“humic-like”, >430 nm) was consistent with chemisorption, removal of DOM emitting at intermediate wavelengths (“humic-like”, 390–420 nm) was consistent with physisorption, and multiple mechanisms were indicated for “protein-like” (<380 nm) DOM. Non-biological mechanisms of DOM removal by aged BAC filters are often assumed to be unimportant; however, these results suggest they are important for some DOM fractions, especially during periods of reduced microbial activity.
Water impactBiologically activated carbon (BAC) filters combine biological and non-biological (physisorption, chemisorption) processes to remove dissolved organic matter (DOM). In order to decouple and investigate these mechanisms, temperature-dependent responses were investigated for various DOM fractions. Experimental results suggest that even in aged BAC filters, non-biological mechanisms occur and may dominate at times of low microbial activity. |
1. Introduction
Dissolved organic matter (DOM; organic material that passes through a 0.45 μm filter) is present in all-natural waters where it is formed from various complex biotic and abiotic reactions affecting organic molecules from a range of sources.1–3 DOM is undesirable in drinking water production and distribution since it forms harmful disinfection by-products (DBPs) during disinfection with chlorine, fuels bacterial regrowth in the distribution system, and can cause taste- and odor-related problems.4,5 Adsorption onto granular activated carbon (GAC) in fixed-bed filters is a common treatment step for removing DOM from drinking water. GAC adsorbs DOM and organic micropollutants to a degree that is influenced by factors like DOM concentration and type, presence of other organic micropollutants, pH, ionic strength and the age and surface properties of the GAC.6–8During the operation of fixed-bed GAC filters, the filter media gradually reaches a state of saturation whereby essentially all available adsorption sites are occupied. A naturally-occurring biofilm starts to develop on the porous GAC surface. Microbes in the biofilm utilize organics accumulated on the surface as a food source, prolonging the service life of the fixed-bed GAC filter through its conversion to a biological activated carbon (BAC) filter. BAC filters preferentially reduce biodegradable organic matter, including some organic micropollutants via a complex coexistence of ad- and desorption and biodegradation processes.9,10 Since these processes are temperature-dependent, for treatment plants located in temperate and polar regions where there is a large seasonal temperature variation, the processes controlling BAC-mediated removal of DOM would be expected to vary seasonally.
Four processes describe the removal mechanisms for DOM in BAC filters under different temperature regimes. First, physisorption is a physical process that is relatively weak in nature, exothermic and potentially reversible. Thermodynamic considerations predict that physisorption is favored by decreasing temperature.11 Second, chemisorption involves chemical reactions between the adsorbate and the surface functional groups on BAC. Chemisorption is rather strong in nature and typically irreversible,11 and chemisorbed species tend to accumulate on the BAC surface reducing its adsorption capacity. Higher temperatures favor chemisorption (until saturation point) as this provides the activation energy required to form adsorbate–adsorbent bond.6,12 Third, biodegradation occurs when microbes use the surfaces provided by GAC to metabolize biodegradable substances in their surroundings. Biological degradation is governed by enzyme activity, which increases with temperature up to a species-dependent tolerance limit.13 Finally, desorption from BAC can be a source of reversibly-attached DOM if there is a lower concentration of DOM in the water than on the BAC surface.14
It is difficult to distinguish between DOM removal processes in practice since they occur simultaneously. Furthermore, DOM is a complex mixture that in treatment plants, is usually assessed by monitoring its overall abundance using bulk parameters like color, total/dissolved organic carbon (TOC/DOC) or UV absorbance at 254 nm (UV254).15,16 However, such bulk indicators do not distinguish between different DOM fractions, although for example, lower-molecular-weight DOM fractions are known to be more efficiently removed by adsorption, and protein-like components, known to be removed more effectively by biodegradation.17,18 Lower-molecular-weight “protein-like” fractions can be distinguished from bulk DOM sensitively and accurately using fluorescence spectroscopy.17,19 However, to the best of our knowledge, fluorescence spectroscopy has yet to be used to investigate the mechanisms controlling the removal of different DOM fractions at different temperature in BAC filters.
The goal of this study was to evaluate the interaction of DOM with BAC filters in drinking water treatment. Biological removal is thought to be the dominant mechanism; however, in this study two additional removal mechanisms (physisorption and chemisorption) and one release mechanism (desorption) were considered.
The specific aims were to:
1. Identify the most likely removal mechanisms of different DOM fractions through their temperature-dependent behavior.
2. Identify the susceptibility of different DOM fraction to back-diffusion during times of rapidly changing operational conditions.
3. Evaluate fluorescence spectroscopy as a tool to identify removal mechanisms and assess back-diffusion of different DOM fractions.
2. Materials and methods
BAC filter media
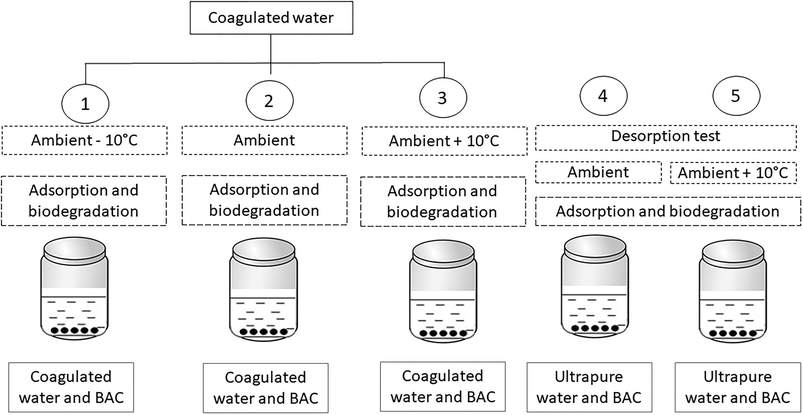
Batch tests were performed using full-scale BAC filter media obtained from Lackarebäck drinking water treatment plant (DWTP), which is the largest plant supplying water to the city of Gothenburg, Sweden. Filter material was collected separately for each experiment on five occasions between July–October 2018 from a single randomly-selected filter that had been in operation for over three years. This filter contained coal-based GAC (Filtrasorb TL 830; Chemviron Carbon) with an iodine number of 900 mg g−1 operated at a contact time of 15–20 min and a surface load of 3.9–4.4 m h−1 with a filter depth of 0.9–1.0 m. Compared to new filter material, the three year old BAC filter media had a lower BET surface area (657.4 m2 g−1vs. of 1039 m2 g−1 for old vs. new) and low pore volume (0.30 cm3 g−1, p/p0: 0.990 vs. 0.63 cm3 g−1, p/p0: 0.990), respectively. Fresh BAC was collected at the beginning of each experiment and at the same time interval after backwashing (three days), as a slurry from the top 5 cm of the bed. After removing excess slurry water, the filter material was immediately homogenized and used within one day of sampling.Experimental design
Experiments (Fig. 1, Table 1) were performed by adding 0.4 g of BAC (wet weight) to 35 mL of settled water in replicate flasks (n = 5 replicates) and placed on a shaker table in a temperature-controlled room. DOM concentration was measured before and after exposing the water to the BAC for 20 hours. The kinetics of adsorption of methylene blue onto the BAC filter material was investigated prior to the DOM sorption experiments. In all tests, the steady-state equilibrium was reached within 20 hours.| Experiment | Season | Treatment | Temperature | Parameters |
|---|---|---|---|---|
| a Desorption test with ultrapure water. | ||||
| 1 | Summer | Ambient −10 °C | 10 °C | Fluorescence |
| Absorbance | ||||
| 2 | Summer | Ambient | 20 °C | Fluorescence |
| Absorbance | ||||
| Fall | 10 °C | Fluorescence | ||
| Absorbance | ||||
| DOC | ||||
| 3 | Summer | Ambient +10 °C | 20 °C | Fluorescence |
| Absorbance | ||||
| Fall | 30 °C | Fluorescence | ||
| Absorbance | ||||
| DOC | ||||
| 4a | Fall | Ambient | 10 °C | Fluorescence |
| Absorbance | ||||
| DOC | ||||
| 5a | Fall | Ambient +10 °C | 20 °C | Fluorescence |
| Absorbance | ||||
| DOC | ||||
The experiments were performed in summer and fall (Table 1) to allow for both seasonal changes in water quality and seasonal changes in microbial community composition. Between these two seasons there was a large drop in ambient temperature (from 20 °C in summer to 10 °C during fall) and slight change in incoming water quality (DOC increased from 3.8 to 4.8 mg L−1 and UV254 from 0.033 to 0.038 cm−1). In each experiment, the ambient temperature at the treatment plant was designated as the reference temperature and experiments were run by increasing and/or decreasing water temperature by 10 °C relative to the reference temperature (experiment 1: ambient −10 °C, experiment 2: ambient, experiment 3: ambient +10 °C). Experiment 1 was performed only in summer while experiments 2 (ambient) and 3 (ambient +10 °C) were repeated for both summer and fall. The highest water temperature tested (30 °C) exceeds the maximum water temperature for water in the DWTP (up to 24 °C in summer) and slightly exceeds the projected maximum temperature under climate change (25–28 °C),20 but is well below the maximum temperature for enzyme functioning in mesophilic microbes (<45 °C).21 Our motivation for experimenting a larger temperature range was in order to be confident in detecting significant differences between treatments.
In experiment 4 (ambient) and 5 (ambient +10 °C), fresh BAC material (0.4 g) was placed in 35 mL ultrapure water at 10 °C and 20 °C for 20 hours, allowing investigation of the effect of a negative concentration gradient.
Dissolved organic matter characterization
Samples (N = 132) were filtered through pre-washed 0.45 μm membrane filters (cellulose acetate). DOM absorbance and fluorescence was measured using a Horiba Aqualog spectrophotometer. Fluorescence excitation-emission matrixes (EEMs) were obtained in a 1 cm cell by scanning the excitation wavelengths from 240–500 nm while emission was detected from 250–650 nm. At the same time, absorbance measurements were obtained corresponding to the fluorescence excitation wavelengths. Processing of fluorescence data followed established methodologies.22 Briefly, this included spectral correction and blank subtraction to remove Raman and Rayleigh scatter as well as correction for primary and secondary inner filter effects. Fluorescence intensities were normalized to the area under the water Raman peak at 350 nm thereby converting fluorescence to Raman units (R.U.).DOC was measured using the high-temperature catalytic combustion method.23 Filtered samples were measured using a Shimadzu TOC-VCPH carbon analyzer. DOC concentrations were calculated using a five-point calibration curve of potassium phthalate standard solutions (1.0–10.0 mg C L−1). DOC measurements are available for the fall experiments 2 and 3 as well as for experiment 4 and 5.
Data analysis
The underlying components of fluorescent DOM measured in 132 samples were isolated with Parallel Factor Analysis (PARAFAC) using the drEEM toolbox.24 Models with four to seven components and non-negative loadings and scores were explored and cross-validated. Ultimately, a split-half validated seven-component PARAFAC model with an explained variance of 99.9% and a core consistency of 0.2% was found to best represent the data set. The obtained spectra representing independently-varying fluorescence fractions were compared to previously published studies using the OpenFluor database.25Concentrations of fluorescent DOM fractions at different temperature were determined relative to initial settled water concentrations. Thus, a relative DOM concentration close to 1 indicated zero removal, values >1 indicated release and <1 indicated removal by BAC filter material.
One-way analysis of variance (ANOVA) was conducted to investigate effects of temperature on fluorescence intensities. Mean values and standard deviation of measurements (n = 5) were calculated for each experiment and statistical significance was determined by calculating p-values at a significance level of α = 0.05 using MATLAB® (v9.6, R2019a).
3. Results and discussion
DOM composition
The initial settled water (pH 6.3–6.7) had low UV absorbance (approx. 0.046 ± 0.02 cm −1) and a SUVA value between 0.97 and 1.2 L mg−1 m−1. This indicates that the initial settled water mainly consisted of DOM with low hydrophobicity and low molecular weight which would be difficult to remove by conventional water treatment.15,26The PARAFAC model of the fluorescence datasets featured seven fluorescence components; these had emission maxima near 430, 390, 460, 520, 420, 320 and 340 nm (Fig. 2). The fluorescence components are henceforth referred to according to their emission maximum, e.g. F430 refers to the component with an emission peak near 430 nm. The first five components (F430, F390, F460, F520 and F420) were dominated by visible-wavelength emission while F320 and F340, emitted light in the UVA region (300–380 nm). Visible wavelength fluorescence components are usually described as “humic-like” components in published literature. Fluorescence components similar to F320 and F340 have been shown to correlate with tryptophan- and tyrosine concentrations27,28 and are typically referred to as “protein-like” in published literature. Fluorescence components similar to F320 have also been identified in soluble microbial products produced during the microbial degradation of DOM.29
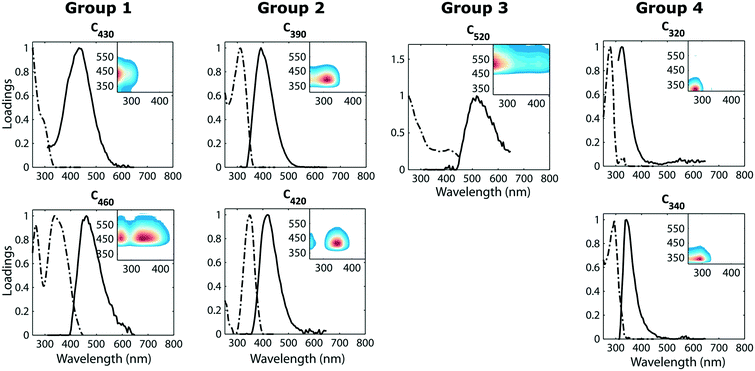 | ||
| Fig. 2 Spectral properties of the seven independent fluorescence components. Inserts in each plot show fluorescence fingerprints (the outer product of emission and excitation spectra). | ||
Comparison with the OpenFluor database revealed that all identified fluorescence components were statistically congruent to published spectra in the database (Tucker congruence exceeding 0.95 for both excitation and emission spectrum). Matches across diverse aquatic environments were found, including when compared to samples from the Baltic sea (F320 with C5 in Stedmon et al.30), recycled wastewater, (F340 with C4 in Murphy et al. (ref. 1)), and drinking water (F390 with C2 in Stedmon et al.29).
Temperature dependence of BAC performance
| Groups | Components | Temperature behaviors | Possible mechanism |
|---|---|---|---|
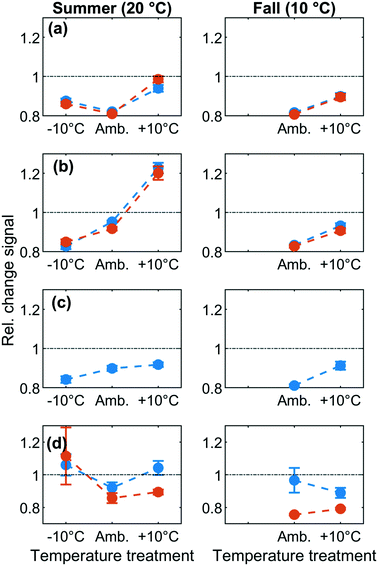
| Group 1 | F 430, F460 | Removal of DOM reached an equilibrium point above which removal declined. | Chemisorption |
| Showed strong temperature effect. | |||
| Group 2 | F 390, F420 | Less DOM removal at elevated temperature (exothermic reaction). | Physisorption |
| Showed strong temperature effect. | |||
| Group 3 | F 520 | Weakly influenced by temperature. | Weak chemisorption |
| Group 4 | F 320, F340 | F 320 removed at the temperature the microbial community acclimatized at. | Chemisorption |
| F 340 did not show any temperature-dependent pattern. |
For group 2 components (F390 and F420), removal decreased with increasing temperature and resulted in a release of group 2 components at ambient +10 °C during summer but not during fall (Fig. 3b). Relative fluorescence was lowest (and removal was greatest) at 10 °C with C/C0 = 0.8 in both summer and fall and decreased to C/C0 = 1.2 (release) at 30 °C (Fig. 3b). This suggests that the predominant removal mechanism for group 2 components was an exothermic process, probably physisorption. Note that the observed removal trend is exactly opposite to what would be expected if removal had been due to a biological process.34
Group 3 consisted solely of component F520. F520 fluorescence responded weakly to temperature changes in both summer and fall experiments, following a similar pattern as group 2 but with a smaller range (C/C0 = 0.8–0.9) (Fig. 3c). It has been hypothesized that F520 tends to be associated with DOM of large molecular-size relative to the remaining fluorescent fractions.35,36 Previous studies have established that large molecular weight DOM is poorly removed by microbes since these compounds are not easily transported across the cell membrane and cannot be attacked by metabolic enzymes.37,38 Our results suggest that weakly exothermic physisorption may be the dominant removal mechanism for this DOM fraction. However, it should be noted that fluorescent DOM tracks a large number of molecular species.36,39 The resulting broad molecular size distributions thus hinder the assignment of a distinct size to each fluorescent fraction.40,41 The exact extent to which molecular size influenced the temperature dynamics of group 3 thus remains unclear.
Group 4 (F320 and F340) “protein-like” components showed limited and variable response to temperature changes in summer and fall experiments (Fig. 3d). Earlier studies reported decreasing protein-like fluorescence through BAC filters, presumably due to microbial degradation,31,42,43 although microbial degradation can also produce this signal.29,44,45 In agreement with this inconsistent picture, we found no statistically significant effects of temperature on group 4 components. Warmer temperatures promote faster biological degradation32,33 and would lead to increased removal and lowered C/C0 for protein-like DOM. Additionally, biological degradation produces microbial protein-like by-products.46 However, compared to other components the abundance of group 4 components was highly variable between replicates. It cannot be excluded that the observed patterns represent the sum of simultaneous microbial uptake and release. Additionally, given the relatively short duration of our experiments (20 hours), we suspect that microbial activity alone would not have led to the release of complex metabolites at the relatively high observed levels (20–25% increase above background fluorescence). Furthermore, high variability is also consistent the greater difficulty of measuring, modeling, and interpreting fluorescence at protein-like wavelengths.47 A diverse group of phenolic compounds, freely dissolved amino acids, or amino acids in peptides may account for the fluorescence at these wavelengths; their varying fluorescence efficiencies48 and changing abundance between samples may be responsible for larger variance in signals between replicates. It was therefore not possible to unambiguously attribute a primary mechanism to the dynamics of group 4 fluorescence components.
Since fluorescence was measured at 10 °C and 20 °C in both summer and fall, it is possible to compare the removal of DOM fluorescence between seasons at the same temperature. For group 2 and 3 fluorescence components, the fraction removed was consistent between seasons at both temperatures (approx. 8 ± 3% at 10 °C and 5 ± 2% at 20 °C, Fig. 3). Also, for F340 in group 4, removal was very similar at the same temperature between seasons, while for F320 in group 4, a seasonal difference was observed at 10 °C only. Only for group 1 components was there a clear difference between seasons, whereby 20% removal was observed at 10 °C in fall but at 20 °C in summer, i.e. only in experiments performed at the ambient water temperature. This might indicate that biodegradation contributed to removing group 1 components, but only when microbes were incubated at their original water temperature.
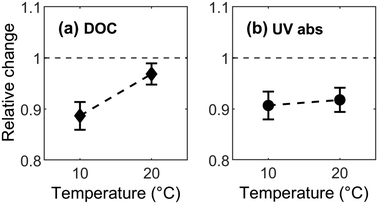
In this study, bulk DOM abundance in terms of DOC measurements available for fall experiments (ambient temperature at 10 °C) facilitate comparisons with previous studies. The average relative concentration (C/C0) of DOC was 0.9 ± 0.1 at 10 °C and increased to 0.98 ± 0.1 at 20 °C indicating that DOC removal decreased from 10% to 2% along with a 10 °C increase in temperature. The observed removal rate at 10 °C is similar to previously reported values of 8–14% in pilot-scale experiments performed on BAC filters receiving coagulated/flocculated/sand-filtered incoming water.10,51 Because DOC removal by BAC decreased with increasing temperature, the dominant removal mechanism for bulk DOC was exothermic in nature (Fig. 4a), consistent with physisorption and confirming the presence of remaining adsorption capacity in the BAC filter material. This result deviates from the general consensus that, after operating for a certain number of years (>3 years in this study), the adsorption capacity of a GAC filter is reduced to effectively zero and all further removal is attributable to biological processes.52–56 The DOC data also suggest that different types of continuously-ongoing regeneration mechanisms within the filter may have freed up previously occupied adsorption sites for physisorption.
In contrast to bulk DOC, UV-absorbing DOM did not show a significant response to changing temperature ([F (3, 18) = 1.15, p = 0.35], Fig. 4b). This differs from results in the study by Lohwacharin, Yang et al. 2014 (ref. 57) where old (>6 years) BAC filters released aromatic UV absorbing DOM fractions and specific UV absorbance (SUVA) increased slightly from 0.9 to 1.2 L mg−1 m−1 with temperature. However, this slight shift in SUVA value did not alter character of DOM; since values remained well below 2 L mg−1 m−1 indicating low average aromaticity.58 The insignificant effect of temperature on the removal of aromatic DOM as measured by UV254via BAC filter in our study is noteworthy. Fluorescence indicates that some fractions are significantly influenced by temperature, but this cannot be tracked using UV absorbance because it is less sensitive and less specific, i.e. it only shows their combined abundance.
DOM desorption from BAC
A recent study by Di Tommaso et al. (2019)59 measured aromatic fractions at much higher concentrations in biofilm than in the water phase. This implies that biofilm can accumulate a large portion of aromatic organic material, and as a result, the concentration of aromatics in the water phase could be much lower. Desorption from activated carbon is an important mechanism affecting BAC and GAC performance when operating conditions change suddenly.14 Desorption can occur by two mechanisms: displacement and/or back-diffusion. Displacement occurs when adsorbed compounds are displaced by compounds with a higher adsorption affinity, releasing the less adsorptive compounds back into solution. Back-diffusion occurs when the concentration of DOM in water surrounding the BAC material decreases rapidly. The concentration gradient is addressed by diffusion of compounds back into solution.60 While displacement may occur constantly during the operation of a BAC filter system, back-diffusion is most likely only noticeable during times of sudden changes in operating conditions, e.g. following rainfall events or clean water backwashing during which DOM concentrations in the water phase decrease rapidly.Desorption due to back-diffusion was evaluated in this study by placing BAC filter material in contact with ultrapure water. All but components F340 and F520 showed a significant release of DOM at 10–20 °C, with a greater release at 20 °C than at 10 °C (p < 0.001, Fig. 5). The release of DOM was greatest for long-wavelength humic-like fluorescence components F430, F390, F460 and F420. This is consistent with increased diffusion rate at higher temperature. This release of long emission wavelength humic-like components could present a mechanism that frees previously-occupied adsorption sites and makes them available to other organics. This mechanism would prolong the bed life of GAC by biologically regenerating the biodegradation capacity and chemisorption capacity when the concentration changes rapidly.61
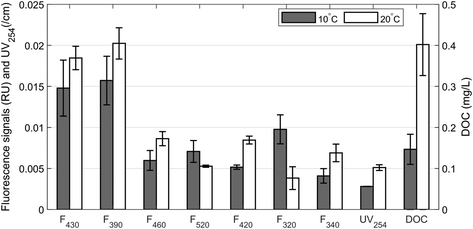 | ||
| Fig. 5 Desorption of fluorescence components, DOC and UV-absorbing DOM from BAC in ultrapure water. Error bars represent the standard error across five replicate samples. | ||
In contrast, “protein-like” F320 and “humic-like” F520 showed less back diffusion with increasing temperature. Since this result is inconsistent with the expected increase in diffusion rate at higher temperatures, it is likely that a combination of mechanisms explains the dynamics of these two fluorescence components with back-diffusion not being the most important of these. For example, the increased number of free adsorption sites may have led to some adsorption of these two components. However, it is presently unclear why this would have been observed only for F320 and F520 and not the other fluorescence components.
Our results for DOC and UV-absorbing DOM are in line with the observations by Suidan et al. (1993),62 who also reported increasing back-diffusion with increasing temperature. This was interpreted as evidence that increased temperatures results in the weakening and ultimate breaking of intermolecular forces between bulk DOM and BAC. However, our use of fluorescence in combination with PARAFAC demonstrates that different mechanisms control adsorption of different DOM subfractions.
Based on the amount and type of DOM that desorbs from a BAC filter due to a lower concentration in water than BAC surface, BAC filters can have beneficial or detrimental effects on the outgoing water quality. For example, in the pilot-scale study done by Qu, F., et al. (2018),63 BAC filters were found to release high molecular weight organics that caused increased fouling potential in the following ultrafiltration process. Similarly, the desorption of a labile fraction like F340 could free up adsorption sites and thus promote the adsorption of more recalcitrant fractions of DOM. Conversely, if absorbable and non-biodegradable refractory organics similar to F430, F390, F460 and F420 are desorbed, adsorption of more labile DOM may be promoted.64 Depending on which fraction is released into the water, the potential for subsequent microbial growth may change depending on changes in the reactivity of DOM in the outgoing water.
The batch-desorption tests in this study are greatly simplified relative to actual conditions affecting BAC filters in a treatment facility. Since these tests do not simulate the plug-flow condition of full-scale BAC filters they cannot be reliably upscaled to predict the amount of DOM that would be removed under full-scale conditions. The lack of evidence for biological removal in this study was surprising and suggests that the biology was either suppressed due to a stress response, or that the experimental conditions were otherwise unfavorable for microbes, for example due to too low encounter rates. This would explain why previous research indicates that BAC filters typically function better at warmer temperatures when microbial activity is high,65,66 while the reverse was typically true in this study. Our batch test results may therefore be most informative about mechanisms that could be observed at full scale during periods of very low biological activity.
Our tests suggest that during summer when surface water temperatures can be around 20–25 °C in Sweden, sorption is reversible for most types of fluorescent DOM and will occur in the presence of a negative concentration gradient. At times when there are rapid increases in temperature, decreases in source water DOM or during backwashing with clean water, the BAC filters could work as a source instead of a sink of organic pollutants. The effect of temperature could also be of importance if another intake depth or a reserve water source is used with a different temperature than the main water source or if a blend of groundwater and surface water is used causing temperature fluctuations. Under climate change, global average temperatures are increasing and precipitation patterns are affected.67 Sweden is experiencing an increase in both temperature and precipitation.68 Such increases affect the occurrence and duration of stratification in lakes and disproportionately affect shallow surface water sources.67,69 The results of this study highlight some ways that changing water temperatures are likely to affect the DOM removal performance of BAC filters.
4. Conclusions
This study investigated the interactions of DOM and BAC filters in drinking water treatment. The temperature dependent behavior of BAC was studied in several batch experiments and the impact of BAC treatment on DOM quality was assessed with fluorescence spectroscopy. Fluorescence spectroscopy was able to provide more in-depth compositional information of different DOM fractions compared to UV254 and bulk DOC; however, for some signals and in some experiments, an unambiguous assignment of mechanisms was difficult due to removal and release occurring simultaneously.For aged BAC, the general assumption is that biological degradation is the dominant factor affecting DOM removal, so that BAC filters tend to function best at higher temperatures when microbial respiration is greatest. However, our results suggest that physical and chemical interaction between BAC and DOM also occur and may be important for some DOM fractions, especially during periods when biological activity is low. Our results further suggest that BAC can be a source of DOM under negative concentration gradients and in times of sudden temperature shifts. Such conditions can be met when water sources or intake depths are changed during the operation of drinking water treatment plants.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This research was funded by the national drinking water research centre DRICKS hosted at Chalmers University of Technology, Swedish Water and Wastewater Association (SVU), Göteborg Stad (Kretslopp och Vatten), Västvatten AB, VIVAB, Trollhättan Energi, Sydvatten and Norrvatten. KRM and UJW acknowledge funding by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS grants 2013-1214 and 2017-00743). The authors thank staff at Lackarebäck DWTP for their valuable and fruitful collaboration, and Inger Kjellberg for sampling assistance.References
- K. R. Murphy, A. Hambly, S. Singh, R. K. Henderson, A. Baker, R. Stuetz and S. J. Khan, Organic matter fluorescence in municipal water recycling schemes: toward a unified PARAFAC model, Environ. Sci. Technol., 2011, 45, 2909–2916 CrossRef CAS PubMed.
- R. L. Malcolm, The uniqueness of humic substances in each of soil, stream and marine environments, Anal. Chim. Acta, 1990, 232, 19–30 CrossRef CAS.
- G. Aiken, D. McKnight, R. Wershaw and P. MacCarthy, Humic Substances in Soil, Sediment, and Water. 1985, Soil Sci., 1986, 142, 323 CrossRef.
- B. W. Dussert and G. R. Van Stone, Biological activated carbon process for water purification, Water Eng. Manage., 1994, 141, 22–24 Search PubMed.
- D. M. Owen, G. L. Amy, Z. K. Chowdhury, R. Paode, G. McCoy and K. Viscosil, NOM characterization and treatability, J. Am. Water Works Assoc., 1995, 87, 46–63 CrossRef CAS.
- R. S. Summers and P. V. Roberts, Activated carbon adsorption of humic substances. II. Size exclusion and electrostatic interactions, J. Colloid Interface Sci., 1988, 122, 382–397 CrossRef CAS.
- T. Karanfil and J. E. Kilduff, Role of granular activated carbon surface chemistry on the adsorption of organic compounds, 1. Priority pollutants, Environ. Sci. Technol., 1999, 33, 3217–3224 CrossRef CAS.
- J. E. Kilduff, T. Karanfil, Y.-P. Chin and W. J. Weber, Adsorption of natural organic polyelectrolytes by activated carbon: a size-exclusion chromatography study, Environ. Sci. Technol., 1996, 30, 1336–1343 CrossRef CAS.
- S. M. Korotta-Gamage and A. Sathasivan, A review: Potential and challenges of biologically activated carbon to remove natural organic matter in drinking water purification process, Chemosphere, 2017, 167, 120–138 CrossRef CAS PubMed.
- P. Servais, G. Billen and P. Bouillot, Biological Colonization of Granular Activated Carbon Filters in Drinking-Water Treatment, J. Environ. Eng., 1994, 120, 888–899 CrossRef CAS.
- M. N. Rashed, in Organic pollutants-monitoring, risk and treatment, IntechOpen, 2013 Search PubMed.
- K. B. Schnelle Jr, R. F. Dunn and M. E. Ternes, Air pollution control technology handbook, CRC press, 2015 Search PubMed.
- B. Schreiber, V. Schmalz, T. Brinkmann and E. Worch, The effect of water temperature on the adsorption equilibrium of dissolved organic matter and atrazine on granular activated carbon, Environ. Sci. Technol., 2007, 41, 6448–6453 CrossRef CAS PubMed.
- Ö. Aktaş and F. Çeçen, Bioregeneration of activated carbon: a review, Int. Biodeterior. Biodegrad., 2007, 59, 257–272 CrossRef.
- J. L. Weishaar, G. R. Aiken, B. A. Bergamaschi, M. S. Fram, R. Fujii and K. Mopper, Evaluation of Specific Ultraviolet Absorbance as an Indicator of the Chemical Composition and Reactivity of Dissolved Organic Carbon, Environ. Sci. Technol., 2003, 37, 4702–4708 CrossRef CAS PubMed.
- J. Fu, W.-N. Lee, C. Coleman, K. Nowack, J. Carter and C.-H. Huang, Removal of disinfection byproduct (DBP) precursors in water by two-stage biofiltration treatment, Water Res., 2017, 123, 224–235 CrossRef CAS PubMed.
- N. M. Peleato, M. McKie, L. Taylor-Edmonds, S. A. Andrews, R. L. Legge and R. C. Andrews, Fluorescence spectroscopy for monitoring reduction of natural organic matter and halogenated furanone precursors by biofiltration, Chemosphere, 2016, 153, 155–161 CrossRef CAS PubMed.
- S. Ciputra, A. Antony, R. Phillips, D. Richardson and G. Leslie, Comparison of treatment options for removal of recalcitrant dissolved organic matter from paper mill effluent, Chemosphere, 2010, 81, 86–91 CrossRef CAS PubMed.
- R. K. Henderson, A. Baker, K. R. Murphy, A. Hambly, R. M. Stuetz and S. J. Khan, Fluorescence as a potential monitoring tool for recycled water systems: A review, Water Res., 2009, 43, 863–881 CrossRef CAS PubMed.
- B. Webb and A. Walsh, Changing UK river temperatures and their impact on fish populations, 2004 Search PubMed.
- B. Bates, Z. Kundzewicz and S. Wu, Climate change and water, Intergovernmental Panel on Climate Change Secretariat, 2008 Search PubMed.
- K. R. Murphy, K. D. Butler, R. G. M. Spencer, C. A. Stedmon, J. R. Boehme and G. R. Aiken, Measurement of dissolved organic matter fluorescence in aquatic environments: An interlaboratory comparison, Environ. Sci. Technol., 2010, 44, 9405–9412 CrossRef CAS PubMed.
- Water Quality: Guidelines for the determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC), BSI, 1997, EN 1484 Search PubMed.
- K. R. Murphy, C. A. Stedmon, D. Graeber and R. Bro, Fluorescence spectroscopy and multi-way techniques, PARAFAC, Anal. Methods, 2013, 5, 6557–6566 RSC.
- K. R. Murphy, C. A. Stedmon, P. Wenig and R. Bro, OpenFluor- an online spectral library of auto-fluorescence by organic compounds in the environment, Anal. Methods, 2014, 6, 658–661 RSC.
- D. M. McKnight, E. W. Boyer, P. K. Westerhoff, P. T. Doran, T. Kulbe and D. T. Andersen, Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity, Limnol. Oceanogr., 2001, 46, 38–48 CrossRef CAS.
- Y. Yamashita and E. Tanoue, Chemical characterization of protein-like fluorophores in DOM in relation to aromatic amino acids, Mar. Chem., 2003, 82, 255–271 CrossRef CAS.
- P. G. Coble, Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy, Mar. Chem., 1996, 51, 325–346 CrossRef CAS.
- S. Hong, T. Xian-chun, W. Nan-xiang and C. Hong-bin, Leakage of soluble microbial products from biological activated carbon filtration in drinking water treatment plants and its influence on health risks, Chemosphere, 2018, 202, 626–636 CrossRef CAS PubMed.
- C. A. Stedmon, S. Markager, L. Tranvik, L. Kronberg, T. Slätis and W. Martinsen, Photochemical production of ammonium and transformation of dissolved organic matter in the Baltic Sea, Mar. Chem., 2007, 104, 227–240 CrossRef CAS.
- S. A. Baghoth, S. K. Sharma and G. L. Amy, Tracking natural organic matter (NOM) in a drinking water treatment plant using fluorescence excitation-emission matrices and PARAFAC, Water Res., 2011, 45, 797–809 CrossRef CAS PubMed.
- J. Lohwacharin, Y. Yang, N. Watanabe, A. Phetrak, H. Sakai, M. Murakami, K. Oguma and S. Takizawa, presented in part at the WA Specialty Conference on NAtural Organic Matter, Costa Mesa, CA, USA, 2011 Search PubMed.
- B. Schreiber, T. Brinkmann, V. Schmalz and E. Worch, Adsorption of dissolved organic matter onto activated carbon—the influence of temperature, absorption wavelength, and molecular size, Water Res., 2005, 39, 3449–3456 CrossRef CAS PubMed.
- M. A. M. Salleh, D. K. Mahmoud, W. A. W. A. Karim and A. Idris, Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review, Desalination, 2011, 280, 1–13 CrossRef CAS.
- S. K. L. Ishii and T. H. Boyer, Behavior of Reoccurring PARAFAC Components in Fluorescent Dissolved Organic Matter in Natural and Engineered Systems: A Critical Review, Environ. Sci. Technol., 2012, 46, 2006–2017 CrossRef CAS PubMed.
- U. J. Wünsch, C. A. Stedmon, L. J. Tranvik and F. Guillemette, Unraveling the size-dependent optical properties of dissolved organic matter, Limnol. Oceanogr., 2018, 63, 588–601 CrossRef.
- H. Kulla, T. Leisinger, A. Cook, R. Hütter and J. Nüesch, Microbial degradation of xenobiotics and recalcitrant compounds, Academic Press, London, 1981, pp. 387–399 Search PubMed.
- B. K. Pramanik, F. A. Roddick and L. Fan, Effect of biological activated carbon pre-treatment to control organic fouling in the microfiltration of biologically treated secondary effluent, Water Res., 2014, 63, 147–157 CrossRef CAS PubMed.
- A. Stubbins, J.-F. Lapierre, M. Berggren, Y. T. Prairie, T. Dittmar and P. A. del Giorgio, What's in an EEM? Molecular signatures associated with dissolved organic fluorescence in boreal Canada, Environ. Sci. Technol., 2014, 48, 10598–10606 CrossRef CAS PubMed.
- U. J. Wünsch, K. R. Murphy and C. A. Stedmon, The one-sample PARAFAC approach reveals molecular size distributions of fluorescent components in dissolved organic matter, Environ. Sci. Technol., 2017, 51, 11900–11908 CrossRef PubMed.
- C. Romera-Castillo, M. Chen, Y. Yamashita and R. Jaffé, Fluorescence characteristics of size-fractionated dissolved organic matter: implications for a molecular assembly based structure?, Water Res., 2014, 55, 40–51 CrossRef CAS PubMed.
- S. Peldszus, C. Hallé, R. H. Peiris, M. Hamouda, X. Jin, R. L. Legge, H. Budman, C. Moresoli and P. M. Huck, Reversible and irreversible low-pressure membrane foulants in drinking water treatment: Identification by principal component analysis of fluorescence EEM and mitigation by biofiltration pretreatment, Water Res., 2011, 45, 5161–5170 CrossRef CAS PubMed.
- F. Chen, S. Peldszus, A. M. Elhadidy, R. L. Legge, M. I. Van Dyke and P. M. Huck, Kinetics of natural organic matter (NOM) removal during drinking water biofiltration using different NOM characterization approaches, Water Res., 2016, 104, 361–370 CrossRef CAS PubMed.
- W. L. Cammack, J. Kalff, Y. T. Prairie and E. M. Smith, Fluorescent dissolved organic matter in lakes: relationships with heterotrophic metabolism, Limnol. Oceanogr., 2004, 49, 2034–2045 CrossRef.
- N. Hudson, A. Baker, D. Ward, D. M. Reynolds, C. Brunsdon, C. Carliell-Marquet and S. Browning, Can fluorescence spectrometry be used as a surrogate for the Biochemical Oxygen Demand (BOD) test in water quality assessment? An example from South West England, Sci. Total Environ., 2008, 391, 149–158 CrossRef CAS PubMed.
- H. Shen, X. Chen, D. Zhang and H.-B. Chen, Generation of soluble microbial products by bio-activated carbon filter during drinking water advanced treatment and its influence on spectral characteristics, Sci. Total Environ., 2016, 569–570, 1289–1298 CrossRef CAS PubMed.
- K. Murphy, S. A. Timko, M. Gonsior, L. Powers, U. Wünsch and C. A. Stedmon, Photochemistry illuminates ubiquitous organic matter fluorescence spectra, Environ. Sci. Technol., 2018, 52, 11243–11250 CrossRef CAS PubMed.
- U. J. Wünsch, K. R. Murphy and C. A. Stedmon, Fluorescence quantum yields of natural organic matter and organic compounds: Implications for the fluorescence-based interpretation of organic matter composition, Front. Mar. Sci., 2015, 2, 98 Search PubMed.
- P. Laurent, M. Prévost, J. Cigana, P. Niquette and P. Servais, Biodegradable organic matter removal in biological filters: evaluation of the CHABROL model, Water Res., 1999, 33, 1387–1398 CrossRef CAS.
- P. M. Huck, S. Peldszus, J. Haberkamp and M. Jekel, Assessing the performance of biological filtration as pretreatment to low pressure membranes for drinking water, Environ. Sci. Technol., 2009, 43, 3878–3884 CrossRef PubMed.
- K. G. Babi, K. M. Koumenides, A. D. Nikolaou, C. A. Makri, F. K. Tzoumerkas and T. D. Lekkas, Pilot study of the removal of THMs, HAAs and DOC from drinking water by GAC adsorption, Desalination, 2007, 210, 215–224 CrossRef CAS.
- D. Urfer, P. M. Huck, S. D. Booth and B. M. Coffey, Biological filtration for BOM and particle removal: a critical review, J. - Am. Water Works Assoc., 1997, 89, 83 CrossRef CAS.
- D. M. Moll, R. S. Summers, A. C. Fonseca and W. Matheis, Impact of Temperature on Drinking Water Biofilter Performance and Microbial Community Structure, Environ. Sci. Technol., 1999, 33, 2377–2382 CrossRef CAS.
- A. C. Fonseca, R. S. Summers and M. T. Hernandez, Comparative measurements of microbial activity in drinking water biofilters, Water Res., 2001, 35, 3817–3824 CrossRef CAS PubMed.
- S. Velten, F. Hammes, M. Boller and T. Egli, Rapid and direct estimation of active biomass on granular activated carbon through adenosine tri-phosphate (ATP) determination, Water Res., 2007, 41, 1973–1983 CrossRef CAS PubMed.
- F. Hammes, M. Berney, Y. Wang, M. Vital, O. Köster and T. Egli, Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes, Water Res., 2008, 42, 269–277 CrossRef CAS PubMed.
- J. Lohwacharin, Y. Yang, N. Watanabe, A. Phetrak and S. Takizawa, Removal of DOM and AOC in a full-scale advanced water treatment plant: effects of operational periods of BAC filters, Water Sci. Technol.: Water Supply, 2014, 14, 165–172 CAS.
- J. K. Edzwald and J. E. Tobiason, Enhanced coagulation: US requirements and a broader view, Water Sci. Technol., 1999, 40, 63–70 CrossRef CAS.
- C. Di Tommaso, L. Taylor-Edmonds, S. A. Andrews and R. C. Andrews, The contribution of biofilm to nitrogenous disinfection by-product formation in full-scale cyclically-operated drinking water biofilters, Water Res., 2019, 155, 403–409 CrossRef CAS PubMed.
- C. J. Corwin and R. S. Summers, Adsorption and desorption of trace organic contaminants from granular activated carbon adsorbers after intermittent loading and throughout backwash cycles, Water Res., 2011, 45, 417–426 CrossRef CAS PubMed.
- M. Scholz and R. Martin, Ecological equilibrium on biological activated carbon, Water Res., 1997, 31, 2959–2968 CrossRef CAS.
- R. D. Vidic, M. T. Suidan and R. C. Brenner, Oxidative coupling of phenols on activated carbon: impact on adsorption equilibrium, Environ. Sci. Technol., 1993, 27, 2079–2085 CrossRef CAS.
- F. Qu, Z. Yan, H. Wang, X. Wang, H. Liang, H. Yu, J. He and G. Li, A pilot study of hybrid biological activated carbon (BAC) filtration-ultrafiltration process for water supply in rural areas: role of BAC pretreatment in alleviating membrane fouling, Environ. Sci.: Water Res. Technol., 2018, 4, 315–324 RSC.
- S. M. Korotta-Gamage and A. Sathasivan, Potential of a biologically activated carbon treatment to remove organic carbon from surface waters, Int. Biodeterior. Biodegrad., 2017, 124, 82–90 CrossRef CAS.
- H. Wang, L. Ho, D. M. Lewis, J. D. Brookes and G. Newcombe, Discriminating and assessing adsorption and biodegradation removal mechanisms during granular activated carbon filtration of microcystin toxins, Water Res., 2007, 41, 4262–4270 CrossRef CAS PubMed.
- A. Andersson, P. Laurent, A. Kihn, M. Prévost and P. Servais, Impact of temperature on nitrification in biological activated carbon (BAC) filters used for drinking water treatment, Water Res., 2001, 35, 2923–2934 CrossRef CAS PubMed.
- I. Delpla, A.-V. Jung, E. Baures, M. Clement and O. Thomas, Impacts of climate change on surface water quality in relation to drinking water production, Environ. Int., 2009, 35, 1225–1233 CrossRef CAS PubMed.
- P. Lind and E. Kjellström, Temperature and precipitation changes in Sweden; a wide range of model-based projections for the 21st century, SMHI, 2008 Search PubMed.
- A. L. Solheim, K. Austnes, T. E. Eriksen, I. Seifert and S. Holen, Climate change impacts on water quality and biodiversity, Background Report for EEA European Environment State and Outlook Report, 2010 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |