Glyphosate and AMPA removal from water by solar induced processes using low Fe(iii) or Fe(ii) concentrations
Abstract
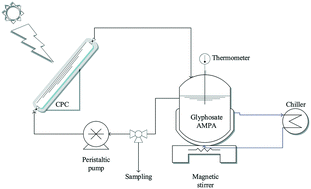
A solar photo-Fenton-like (SPF-like) process is explored for the removal of 1 mg L−1 glyphosate and its main degradation by-product, aminomethylphosphonic acid (AMPA), from Milli-Q water by means of low Fe(III) concentrations (0.6–2 mg L−1) at pH = 2.8 and variable H2O2 concentrations at the laboratory scale. The research is focused on glyphosate and AMPA oxidation, which present similar toxicity patterns. A 1 mg L−1 glyphosate solution requires 5–6 h of the SPF-like process to be degraded when a minute Fe(III) concentration (0.6 mg L−1) is used in acidic water. Glyphosate abatement time is diminished to 2 h when the Fe(III) concentration is increased to 2 mg L−1. At pH levels above 2.8, the herbicide is partially adsorbed onto the colloids of iron oxy(hydroxide) compounds. AMPA requires a higher oxidative power than glyphosate to be degraded, and more than 6 h of solar treatment are needed, using 10 mg L−1 h−1 H2O2 and 2 mg L−1 Fe(III) at acidic pH. Solar photo-Fenton (SPF), using 4 mg L−1 Fe(II) and 10 mg L−1 h−1 H2O2 at pH = 2.8, must be applied to achieve practically total AMPA removal in 6 h of irradiation. SPF-like and SPF treatments led to 70% and 80% mineralization, respectively, under the best operational conditions. This work demonstrates that SPF-like and SPF at low Fe(III) or Fe(II) concentrations are effective treatments for the removal of glyphosate from water at acidic pH. Continuous addition of H2O2 is required for AMPA abatement.
- This article is part of the themed collections: Celebrating Latin American Talent in Chemistry and Best Papers 2019 – Environmental Science: Water Research & Technology


 Please wait while we load your content...
Please wait while we load your content...