Open Access Article
Open Access ArticleOxidation of ammonium by Feammox Acidimicrobiaceae sp. A6 in anaerobic microbial electrolysis cells†
Melany
Ruiz-Urigüen
 a,
Daniel
Steingart
a,
Daniel
Steingart
 b and
Peter R.
Jaffé
b and
Peter R.
Jaffé
 *a
*a
aDepartment of Civil and Environmental Engineering, Princeton University, Princeton, New Jersey, USA. E-mail: jaffe@princeton.edu
bDepartment of Mechanical and Aerospace Engineering, Princeton University, Princeton, New Jersey, USA
First published on 26th July 2019
Abstract
Anaerobic ammonium oxidation under iron reducing conditions, also referred to as Feammox, can be carried out by the recently isolated Acidimicrobiaceae sp. A6 (A6). Ammonium is a common water pollutant which is typically removed by nitrification, a process that exerts a high oxygen demand in waste treatment systems. A6 oxidizes ammonium anaerobically using ferric iron [Fe(III)] as an electron acceptor and has also been shown to be an electrode (anode) colonizing bacterium. Results presented here demonstrate that A6, in a pure or enrichment culture, can thrive in microbial electrolysis cells (MECs) by oxidizing ammonium, while using the anode as an electron acceptor. Results also show that current production and ammonium removal increase with the concentration of 9,10-anthraquinone-2,6-disulfonic acid (AQDS), a soluble electron shuttling compound, which is especially noticeable for the pure A6 culture. Electron microscopy of the anode's surface reveals attached cells in the pure culture MEC; however, over the time of operation there is no formation of a biofilm and the majority of cells are in the bulk liquid, explaining the need for AQDS. Maximum coulombic efficiencies of 16.4% and a current density of 4.2 A m−3 were measured. This is a first step towards the development of a Feammox bacteria-based bioelectrochemical system for anaerobic ammonium oxidation while reducing electrodes instead of Fe(III).
Water impactCoupling the Feammox process to MECs to facilitate the removal of ammonium using the anode as an electron acceptor, instead of having to add solid-phase ferric iron to a reactor, is a first step for the development of new Feammox-based anaerobic methods for ammonium oxidation, which, if successful, are likely to result in significant energy savings over traditional aerobic nitrification methods. |
1. Introduction
Ammonium (NH4+) can accumulate in soil and water1 and can be detrimental to the environment, particularly water systems. Nitrification, the conversion of NH4+ to nitrite (NO2−) and nitrate (NO3−), is the most extensively used method to oxidize NH4+ in engineered systems. However, nitrification is energetically intensive as it requires oxygen inputs, which can account for a substantial amount of energy usage in wastewater treatment plants during the operation of aerators.2,3 To lower the energy consumption for wastewater treatment, anaerobic oxidization of NH4+ is a worthwhile endeavor. Anammox oxidizes NH4+ anaerobically by coupling it to NO2− reduction, but some aeration is still required to form the needed NO2−. Anaerobic NH4+ oxidation under iron reducing conditions is commonly referred to as Feammox. The oxidation of NH4+via Feammox to NO2− (ref. 4–8) occurs in the absence of molecular oxygen, which makes it an attractive candidate for the development of an energy efficient NH4+ removal method. However, it requires iron oxides [Fe(III)] as electron acceptors in a stoichiometric ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ref. 4 and 9) (eqn (1)–(3)).
1 (ref. 4 and 9) (eqn (1)–(3)).| NH4+ + 2H2O → NO2− + 8H+ + 6e− | (1) |
| 3Fe2O3·05H2O + 18H+ + 6e− → 6Fe2+ + 10.5H2O | (2) |
| NH4+ + 3Fe2O3·05H2O + 10H+ → NO2− + 6Fe2+ + 8.5H2O | (3) |
Iron is abundant in the environment and thus Feammox can be enhanced in systems such as constructed wetlands to treat some wastewaters.10 However, adding a stoichiometric amount of Fe(III), shown in eqn (1), to wastewater treatment reactors to remove NH4+via the Feammox reaction is not practical because the large amount of iron oxides required results in the accumulation of iron phases over time in the reactor, which requires removal and disposal. To implement the Feammox process for large scale or long-term continuous-flow reactor applications, the requirement of having to add a solid Fe(III) phase needs to be addressed, and an electrode is a suitable option as it can act as the electron acceptor substituting the need for Fe(III) addition.
Electroactive bacteria have been used in bioelectrochemical systems (BES) to extract energy from different types of electron donors and transfer the electrons to the electrodes (anode).11,12 The electron transfer process can be direct or aided by electron shuttles7,13 and results in the production of low-density electrical currents.12 Feammox is a microbially mediated process known to be carried out by an Actinobacterium named Acidimicrobiaceae sp. A6 (A6),7 which is an iron reducer, a feature present in many electroactive bacteria. Experiments to date have shown that A6 grows on solid Fe(III) phases such as ferrihydrite, but it does not grow on dissolved Fe(III) such as ferric citrate or ferric chloride.7 Furthermore, it has been shown that A6 is an electrode colonizing bacterium linked to current production using either a natural redox potential gradient such as in the case of electrodes placed in wetlands where the anode is placed in the more reduced sediment and the cathode is placed in the more oxidized sediment, or in constructed systems when an external potential is applied.14 Harnessing A6's ability to use electrodes in BES such as microbial electrolysis cells (MECs) may result in the removal of NH4+, bypassing the need of supplying an electron acceptor such as Fe(III) (eqn (4)).
| NH4+ + 2H2O + 6e− → NO2− + 3H2 + 2H+ | (4) |
Although other researchers have proposed that NH4+ can be oxidized to N2via Feammox,8 studies with the pure A6 culture7 and with an A6 enrichment culture using acetylene and N-15 labeled N (ref. 9) have shown that A6 oxidizes NH4+ to NO2− as shown in eqn (1).
MECs utilize a small external voltage (0.2–0.8 V) for overcoming the thermodynamic barrier of electrolysis. The potential difference between the anode and the cathode is enough to drive the electron transfer from the NH4+ oxidation reaction. The possibility of a reaction can be determined by calculating its standard free energy (ΔG°′) applying the Nernst equation (ΔG°′ = −nFΔE°′), where n is the number of electrons transferred during the reaction (n = 6 for NH4+ → NO2−), F is the Faraday constant (96.485 kJ V−1 mol−1), and ΔE°′ is the difference in the potentials between two half reactions, measured in volts (V) 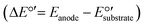 .
. 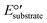 is equal to 0.07 V for NH4+ oxidation to NO2− (see the ESI† for calculation details). Hence, to make the reaction feasible, Eanode needs to be above 0.07 V vs. the standard hydrogen electrode (SHE).
is equal to 0.07 V for NH4+ oxidation to NO2− (see the ESI† for calculation details). Hence, to make the reaction feasible, Eanode needs to be above 0.07 V vs. the standard hydrogen electrode (SHE).
Since we have recently shown that A6 can colonize electrodes in wetland systems and in MECs, and that by doing so current is produced,14 the objective of this study was to build on these results and gain new insights into the bioelectrochemical conditions required for such systems. A specific goal was to determine if pure and enrichment cultures of the Feammox bacterium Acidimicrobiaceae sp. A6 can grow and can carry out anaerobic NH4+ oxidation in the absence of Fe(III) in MECs, and if so, if the rates of NH4+ oxidation and A6 growth in MECs are comparable to those in a batch reactor with Fe(III) as the electron acceptor. For A6 enrichment cultures in MECs, an additional goal was to analyze the overall microbial community to determine if there are other known NH4+ oxidizers present and to get an assessment of the A6 relative abundance in such systems. This was achieved by continuously monitoring current production, measuring NH4+ removal and the A6 biomass concentration in MECs with pure A6 and A6 enrichment cultures. Results presented here are a first step towards the development of a Feammox-bacteria-based system for anaerobic ammonium oxidation in bioelectrochemical reactors in the absence of Fe(III).
Furthermore, it was recently shown that A6 can degrade pollutants such as trichloroethylene (TCE) cometabolically15 as well as defluorinate per and polyfluoroalkyl substances (PFAS) via reductive dehalogenation.16 Hence, showing that one can grow A6 in MECs would, in addition to applications of NH4+ removal, lead to novel methods to utilize this strain for biological degradation of a variety or recalcitrant pollutants in bioelectrochemical reactors without the need to handle solid Fe(III) phases.
2. Materials and methods
2.1 Experimental set-up
MECs were constructed and run in parallel as described by Call and Logan (2011),17 using a stainless steel mesh as the cathode and a graphite plate as the anode since it is chemically stable.18 The headspace of each MEC was purged with an 80% N2 and 20% CO2 gas mixture, and autoclaved. MECs were connected in parallel to a programmable power supply (model 3645A; Circuit Specialists Inc.) with a constant external applied voltage (Vapp) set at 0.3 V. Voltage was recorded hourly with a multimeter (model 2750; Keithley Instruments Inc.) across a 10 Ω resistor placed between the lead connecting the anode and the positive terminal of the power supply. Current (I) was calculated using Ohm's Law (I = V/R), where V is the voltage and R is the resistance. Data are reported as the volumetric current density (Id = A m−3) which was obtained by dividing the current by the liquid volume in the MEC.2.2 Cyclic voltammetry
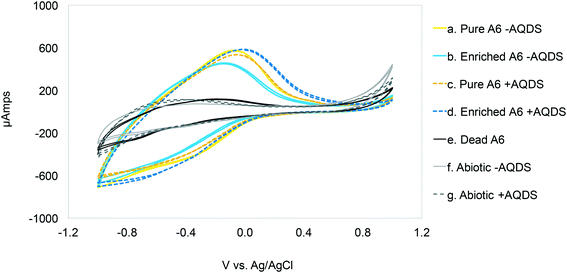
To determine the oxidation peaks for the Feammox reaction in the MECs, cyclic voltammetry (CV) was conducted on the anode, with the cathode as the counter electrode, and a 1 mm thick Ag/AgCl 3.5 M KCl reference electrode (model ET072-1mm, EDaq Inc.) placed between the working and counter electrodes. The applied potential to the MECs was cycled using an Ivium potentiostat, using the Ivium software. Three consecutive scans were conducted, which ranged from −1 V to +1 V at a rate of 1 mV per second. Only the last 2 scans are shown to avoid overcrowding of the figure. CVs were conducted on MECs with live pure A6 and A6 enrichment cultures with and without 9,10-anthraquinone-2,6-disulfonic acid (AQDS), which is an electron shuttling compound, to determine possible effects of AQDS on the system's oxidation peaks. CV was also conducted on MECs with a dead A6 culture with AQDS, and on abiotic control MECs with and without AQDS to confirm that the peaks found were the result of biotic activity from A6.2.3 MEC operating conditions
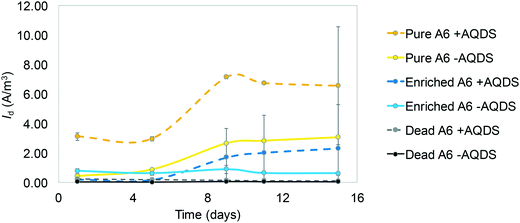
Each MEC was inoculated with an A6 pure or enrichment culture in a Feammox enrichment medium, with a total volume of 8 ml. The cultures were maintained and provided by Dr. Shan Huang, following the protocol described in Huang and Jaffé (2018).7 The medium contained the following: NH4Cl 5 mM, NaHCO3 0.24 mM, KHCO3 0.71 mM, KH2PO4 0.052 mM, MgSO4·7H2O 0.41 mM, CaCl2 0.54 mM, vitamin supplement (ATCC® MD-VS) 0.1 μl l−1, trace element solution as described by Sawayama5 and AQDS 0.15 mM. AQDS was included after determining that its addition facilitated electron transfer to the anode from NH4+ oxidation, enhancing both the amount of ammonium oxidized and hence the current produced (Fig. 3). Additionally, it has been shown that AQDS is required to grow the pure A6 culture when Fe(III) is the electron acceptor,7 while for long-term growth of A6 enrichment cultures, AQDS is not needed.9 Vials contained resazurin (1 mg L−1) as an indicator of anaerobic conditions. The pH of the medium was initially set to 5–5.5 because the Feammox process works best under acidic conditions with pH below 6.3.7,9Replicates of MECs with working Feammox cultures were run for each experiment (n = 2 for pure cultures and n = 4 for enrichment cultures). Three types of controls were set up to confirm that current production and NH4+ removal were the result of biotic activity: 1) MECs with dead bacteria by autoclaving, 2) abiotic MECs with enrichment medium without microbial inoculum, and 3) MECs with live bacteria in Feammox medium without NH4+. Furthermore, positive controls with a live Feammox A6 pure culture were incubated in identical vials without a working anode or cathode, containing Fe(III) in the form of 2-line ferrihydrite as the electron acceptor19 for NH4+oxidation and biomass change comparison. All MECs were placed on a mixing plate at 240 rpm; however, using a mixing plate for this purpose resulted in deterioration over time of the connections in the MECs due to the shaking of the reactors' connections. Therefore, to expand the operational time of the MECs and to avoid noisy readings generated by movement of the whole reactor on the mixing plate, a later set-up of the MECs with the pure culture consisted of magnetic stirring bars placed in each reactor positioned on a stirring plate, such that only the liquid content in each MEC moved due to the mixing. Since mixing regimes can affect any reactor performance, no comparison between experiments with different mixing regimes is done here. Mixing via stirring is only done to expand the operation time of the MEC and to compare its performance against conventional Feammox incubations with Fe(III) as the electron acceptor. When current production decreased, the headspace was flushed with an 80% N2 and 20% CO2 gas mixture to ensure that CO2 was not a limiting factor as A6 is an autotroph, and to remove any H2 formed, as it has been shown that A6 is capable of oxidizing it.7 The coulombic efficiency (CE = CP/CT × 100%) was determined as the percentage ratio between the produced coulombs (C), calculated by integrating the measured current over time 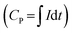 , divided by the theoretical amount of C produced from the amount of NH4+ oxidized to NO2− (CT = ΔNH4+nF), where ΔNH4+ is the change in moles NH4+ measured, n is the amount of electrons produced from NH4+ oxidation to NO2− (6 mM electrons per mM NH4+), and F is the Faraday constant (96
, divided by the theoretical amount of C produced from the amount of NH4+ oxidized to NO2− (CT = ΔNH4+nF), where ΔNH4+ is the change in moles NH4+ measured, n is the amount of electrons produced from NH4+ oxidation to NO2− (6 mM electrons per mM NH4+), and F is the Faraday constant (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485.33 C mol−1).
485.33 C mol−1).
2.4 Chemical analyses
Samples of 1 ml were taken from the MECs at the beginning and end of each experiment. Each sample was filtered using a 0.2 μm pore size syringe filter and used to measure NH4+ and NO2− concentrations in a Dionex™ ICS3000 Ion Chromatograph, with a CS-16 column, a CS-16 guard column, and a CERS 500 (4 mm) suppressor for cations and with an AS-22 column, an AG-22 guard column, and an ASRS 300 (4 mm) suppressor for anions. Iron was analyzed to quantify the small amount of Fe that was transferred with the culture seed to the MECs (ESI,† Table S1). Total Fe was analyzed by adding 100 μl of the MEC or control culture to 4.8 ml of 1 N HCl and 100 μl of 6.25 M NH2OH–HCl, then Fe was quantified photometrically using the ferrozine method,20 as adapted by Komlos and Jaffé.21 Ferrous iron [Fe(II)] was quantified by the direct ferrozine method.2.5 DNA extraction, quantification and microbial community composition
Total genomic DNA was extracted from 5 ml of a working culture of the MECs and from positive live controls at the end of each operational period. DNA was extracted using a FastDNA® spin kit for soil (MP Biomedicals, USA) following the manufacturer's instructions with an additional first step in which the bacteria were concentrated by centrifuging the liquid medium for 10 min at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 relative centrifugal force (RCF); the pellet was resuspended in 500 μl of the supernatant and used as the initial substrate for extraction. Total DNA was eluted in 100 μl of sterile water and its concentrations were measured using Qubit 2.0® (Invitrogen, USA). All DNA samples were preserved at −20 °C until further analysis.
000 relative centrifugal force (RCF); the pellet was resuspended in 500 μl of the supernatant and used as the initial substrate for extraction. Total DNA was eluted in 100 μl of sterile water and its concentrations were measured using Qubit 2.0® (Invitrogen, USA). All DNA samples were preserved at −20 °C until further analysis.
Quantification of A6 in the pure culture was carried out via qPCR using an Applied Biosystems StepOnePlus real-time PCR system by amplifying a section of the 16S rRNA gen using primer set 1055F/1392R (1055F, 5′-ATGGCTGTCGTCAGCT-3′; 1392R, 5′-ACGGGGCGGTGTGTAC-3′). Each qPCR mixture (20 μl) was composed of 10 μl of SYBR Premix Ex Taq II 2X (TaKaRa, Japan), 0.8 μl of each forward and reverse primer at 10 μM, and the DNA template. Thermal cycling was initiated with 30 s at 95 °C, followed by 40 cycles, each cycle consisting of 5 s at 94 °C, 30 s at 55 °C, and 30 s at 70 °C. Each qPCR assay was run in triplicate for each sample and included negative controls and a standard curve; the latter consisted of serial dilutions of known numbers of copies of DNA.
In order to determine the microbial community composition, sequencing and phylogenetic analysis was performed by Novogene (Beijing, China) as follows: from total genomic DNA, the variable region V4 of the 16S rRNA gene was amplified using the primer set 515F/806R (51 F: 5′-GTGCCAGCMGCCGCGGTAA-3′/806R: 5′-GGACTACHVGGGTWTCTAAT-3′) with a barcode following the method of Caporaso et al. (2011).22 All PCR reactions were carried out with a Phusion® High-Fidelity PCR master mix (New England Biolabs). Quantification and qualification of PCR products was carried out by electrophoresis on 2% agarose gel. The resulting amplicons were pooled, purified, and quantified. Sequencing libraries were generated using a TruSeq® DNA PCR-free sample preparation kit (Illumina, USA) following the manufacturer's protocol and index codes were added. The library quality was assessed on a Qubit@ 2.0 Fluorometer (Thermo Scientific) and an Agilent Bioanalyzer 2100 system. Finally, sequencing was performed on an Illumina HiSeq 2500 platform and 250 bp paired-end reads were generated.
Paired-end reads were assembled by using FLASH V.1.2.7.23 Raw reads were processed according to a QIIME V1.7.0 quality controlled process24 and chimeric sequences were filtered out using the UCHIME algorithm.25 A total of 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 969 sequences were obtained which were clustered into operational taxonomic units (OTUs) using UPARSE V7.0.1001.26 Sequences with ≥97% similarity were assigned to the same OTUs. A total of 995 OTUs were produced. A representative sequence for each OTU was screened for taxonomic annotation using the BLAST algorithm against the 2016 NCBI's 16S ribosomal RNA sequences for bacteria and archaea at an e-value of 1e−5. A6's 16S rRNA gene sequence (GenBank 2017 accession number MG589453) was included in NCBI's database for annotation at the family and genus level of the top 100 most abundant OTUs.
969 sequences were obtained which were clustered into operational taxonomic units (OTUs) using UPARSE V7.0.1001.26 Sequences with ≥97% similarity were assigned to the same OTUs. A total of 995 OTUs were produced. A representative sequence for each OTU was screened for taxonomic annotation using the BLAST algorithm against the 2016 NCBI's 16S ribosomal RNA sequences for bacteria and archaea at an e-value of 1e−5. A6's 16S rRNA gene sequence (GenBank 2017 accession number MG589453) was included in NCBI's database for annotation at the family and genus level of the top 100 most abundant OTUs.
2.6 Environmental scanning electron microscopy of the MECs' anode
The graphite plate working as an anode of MECs containing live A6, and one from the autoclaved MEC, both from MECs operated under stirring for over 1 month, were analyzed using an environmental scanning electron microscope (Quanta 200 FE-ESEM), following the instrument's protocol.3. Results and discussion
3.1 Cyclic voltammetry analysis
Cyclic voltammetry (CV) results (Fig. 1) show oxidation peaks for all biotic MECs containing AQDS at −0.03 ± 0.025 V vs. Ag/AgCl (3.5 M KCl, + 205 mV vs. SHE), and for the experiments without AQDS the peak was slightly shifted toward more negative values, −0.11 ± 0.035 V vs. Ag/AgCl. Applying these voltages to calculate the ΔG° for the biotic reactions, using the Nernst equation, results in −60.78 kJ mol−1 with AQDS and −14.47 kJ mol−1 without AQDS. The effect of AQDS on the oxidation peaks does not appear to be ample nor significantly different for the pure or enrichment culture; nonetheless, initial MEC analysis with pure and A6 enrichment cultures (Vapp of 0.7 V) clearly showed that AQDS has a greater effect when added to the pure A6 culture than to the A6 enrichment culture (Fig. 2). These results are consistent with other studies which have demonstrated that electron shuttling compounds, such as natural organic matter, can facilitate electron transfer from the cell to an electron acceptor,27,28 or that certain bacterial cells can enable cell-to-cell electron transfer.29 In the absence of available organic matter, analogues, such as quinones, aid the electron transport between the cell and the final electron acceptor30 and facilitate NH4+ oxidation by aiding electron transfer to the anode (Fig. 3). Therefore, in the absence of natural electron shuttles, AQDS is a good option to facilitate electron transfer, including in BES, as it has been shown that graphite anodes incorporated with microbial oxidants, such as AQDS, can increase their Id performance,31,32 as seen in our system. Given the decreased effect of AQDS on current production in the A6 enrichment culture, it is unlikely that AQDS will be needed in A6 enrichment cultures grown in MECs or other bioelectrochemical reactors for an extended time, since organic matter will accumulate due to cell turnover. In the pure culture MECs, however, a monotonic increase in ammonium removal and current produced with an increasing AQDS concentration was observed (Fig. 3), whereas no current was produced in the presence of AQDS and in the absence of NH4+ (ESI† Fig. S1).Other studies on NH4+ removal using BES inoculated with sludge from wastewater treatment plants reported current oxidation peaks of 0.59 V vs. Ag/AgCl (ref. 33) and 0.53 V vs. Ag/AgCl.34 The conditions used in the mentioned studies, such as a pH value of 7.7 (Feammox reaction requires acidic pH), and the associated microbial community, are different from the ones presented here. Furthermore, Zhan et al. (2014)33 report a microbial community mainly composed of Stenotrophomonas, Nitrosomonas, Comamonas and Paracoccus; there is no mention of Acidimicrobiaceae in their system. Vilajeliu-Pons et al. (2018)34 attribute nitrification in their system to Nitrosomonas, and although they found Actinobacteria, the phyla to which A6 belongs to, the conditions are not optimal for A6, making it inconclusive that the Feammox process might have contributed to NH4+ removal in their experiments. Additionally, oxidation peak values similar to the ones found for the MECs with A6 (−0.08 vs. Ag/AgCl) have been reported for other MEC systems.35 Nonetheless, that system set-up and microbial community composition was different to the one reported here, as it contained organic carbon and higher pH, to promote growth of organisms such as Geobacter spp.35,36
The CV on abiotic and dead controls did not show a peak near the oxidation peak for the biotic reactors. The amplitude of the voltammogram on the reverse scans is believed to be due to a pseudocapacitance behavior of the system. The proposed NH4+ oxidation reaction in the MECs (NH4+ + 2H2O → NO2− + 3H2 + 2H+) supports the increased amplitude of the voltammogram during reverse scanning, because protons can undergo adsorption and desorption prior to H2 formation during scans.37
3.2 MECs with pure Acidimicrobiaceae sp. A6 culture
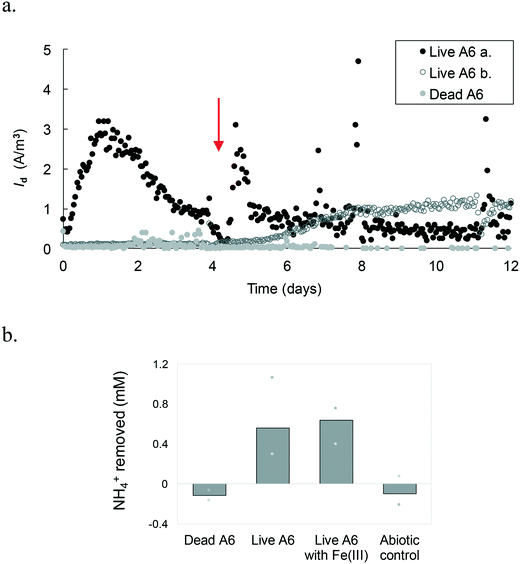
Results show that A6 has the ability to be active in MECs, under constant mixing and with Vapp. Different replicas of the MECs run with the pure A6 culture had Id peaks at different time points (Fig. 4.a). For example, some peaked in the first couple of days, with a maximum Id value of up to 3.2 A m−3, while other live A6 cultures ramped up slower and never peaked; instead, they showed a stable increase of Id and then leveled out. Differences in the performances of MECs' replicas have been reported before, being the most noticeable at the start-up period;38 however, the reason for such behavior needs further analysis. One possible explanation are stochastic processes, such as colonization of the anode's surface, which could have affected the microbial community function.39 Although MECs were operated for 3 weeks, Id data are shown only for a 2-week period, because after that, the connections to the electrodes became loose due to the constant shaking, resulting in noisy data. The MECs with a pure culture had a CE of 4.33%. All control conditions, including MECs with dead A6, abiotic MECs, and MECs with live A6 without NH4+ showed negligible Id (ESI† Fig. S1).All MECs containing the live A6 culture removed on average 0.6 ± 0.25 mM NH4+, which is similar to the amount removed for cultures grown using Fe(III) as the electron acceptor, over the same time period, i.e. 0.63 ± 0.14 mM NH4+. The control conditions showed no removal of NH4+, in contrast a slight increase in NH4+ concentration was detected (+0.11 ± 0.09 mM NH4+) (Fig. 4.b), which could be due to desorption of NH4+ from the solid phase, such as the ammonium sorbed to cell membrane which has an overall negative charge facilitating the transfer of some NH4+ from the initial bacterial culture to the MECs. NO2− concentration measurements in MECs were below the detection limit. Low detection of formed NO2− with respect to NH4+ removed via the Feammox process has been previously reported;7,9 however, a N mass balance in Feammox enrichment cultures was observed when acetylene gas (C2H2) was added,9 which stops the loss of N in the form of N2, by inhibiting the reduction of N2O to N2. Abiotic Fe(II) oxidation by NO2− has been observed under similar conditions to the ones used during the Feammox process and in the MECs,40,41 which results in the reduction of NO2− to different N-gas forms. Hence, a control test incubating NO2− with Fe(II) while tracking the NO2− concentration over time was performed. Results show that NO2− disappeared in the presence of Fe(II) (Fig. S2†). The presence of small amounts of Fe that are transferred to the MECs with the bacterial seed (Table S1†) explains why NO2− was not detected, as it would have reacted with the transferred Fe(II).
Quantification of biomass from MECs with the pure live A6 culture revealed that the A6 number can be sustained as well as when grown as a pure culture with Fe(III) as the electron acceptor over 3 weeks of operation (2.90 × 109 ± 2.8 × 109 copies of DNA per ml in MECs and 3.35 × 109 ± 1.97 × 109 copies of DNA per ml in batch culture with Fe(III)).
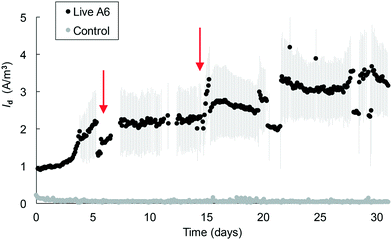
Mixing of the medium in the MECs facilitates transport of NH4+ to cells on the electrode and/or of reduced AQDS to the electrode. However, using a shaker for this purpose resulted in deterioration over time of the connections in the MECs. To expand the operational time of the MECs, magnetic stirring bars were placed in each reactor, which were placed on a stirring plate. This change allowed the operation of MECs with the pure A6 culture for over 1 month, with a continuous increase in Id over time, with up to 4.2 A m−3 (Fig. 5). MECs removed an average of 0.66 ± 0.03 mM NH4+, comparable to pure A6 batch cultures containing Fe(III) as the electron acceptor which removed 0.64 mM NH4+. Both MECs and control batch cultures sustained similar amounts of A6 biomass (2.11 × 109 ± 1.33 × 109 copies of DNA per ml in MECs and 6.97 × 109 copies of DNA per ml in batch culture with Fe(III)). This set-up resulted in a CE of 16.4%.
In MECs operated with the pure A6 culture, Id values of up to 3.2 A m−3 were measured in MECs within 2 weeks, and values of up to 4.2 A m−3 were measured in MECs that operated for over 1 month. These values are on the lower side of Id measured in other MEC systems;42 however, their performance cannot be directly compared since their substrates, microbial communities and reactor configurations are different. The highest coulombic efficiency achieved in the MECs was 16.4% which is similar to values found in other studies (CE 9.6–26.4%)43 but is on the low side for CE values reported for other systems (CE ≥ 23%);44 however, none of those systems are comparable to the ones described here as those other studies contained organic C as their electron donor. The CE values for anaerobic NH4+ removal in MECs from other studies are 32.7–50%,45,34 which are higher than those reported in this study; however their systems also differ from the one described here in that their microbial communities are composed of a high bacterial diversity, many of which are involved in various steps of the N cycle. A low CE means that the electrons from NH4+ are not recovered as current, and this could be due to the use of these electrons for the formation of secondary metabolites46 or mediators that can be stored and used later,43 or to the lack of biofilm formation on the electrode's surface.47 A low CE is also associated with low Vapp, as can be seen from the different Id values measured in MECs with a Vapp value of 0.2 V (Fig. 4, 5 and 6) versus MECs with a Vapp value of 0.7 V (Fig. 2 and 3) which reached higher Id; therefore, applied voltage is a variable that warrants further analysis.
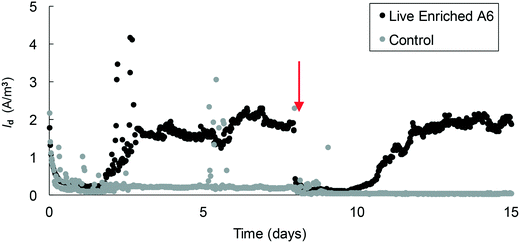 | ||
| Fig. 6 Average current density measured in MECs containing an A6 enrichment culture. The arrow represents the point of headspace flushing and injection of an 80% N2 and 20% CO2 gas mixture. | ||
E-SEM revealed bacterial rod-shaped cells, approximately 1.5–3 μm long by 0.5 μm wide, as described for A6,7 attached to the anode's surface of the MEC with the pure live A6 culture, while no cells could be found on the electrode's surface of the control MEC with dead bacteria (ESI† Fig. S3†). This result is consistent with a previous study in natural and constructed wetlands showing that the A6 population was enhanced on electrode surfaces working as anodes while no A6 enrichment was found on unconnected plates, thus indicating the need for a voltage difference to promote A6 preference for the anode's surface.14 The formation of a biofilm was not observed during the period and operating conditions of the MECs. Quantification of A6 through qPCR showed that the majority of the cells were in the bulk liquid. This explains why the addition of an electron shuttle like AQDS aided NH4+ oxidation and current production, as it facilitated the transfer of electrons between the bacterial cell and the electrode surface. The lack of biofilm formation and the presence of AQDS could have also contributed to the low CE, since some AQDS could have remained in the MECs in its reduced form, without having transferred from the electrode to the anode where it would have produced a current.
3.3 MECs with Acidimicrobiaceae sp. A6 enrichment culture
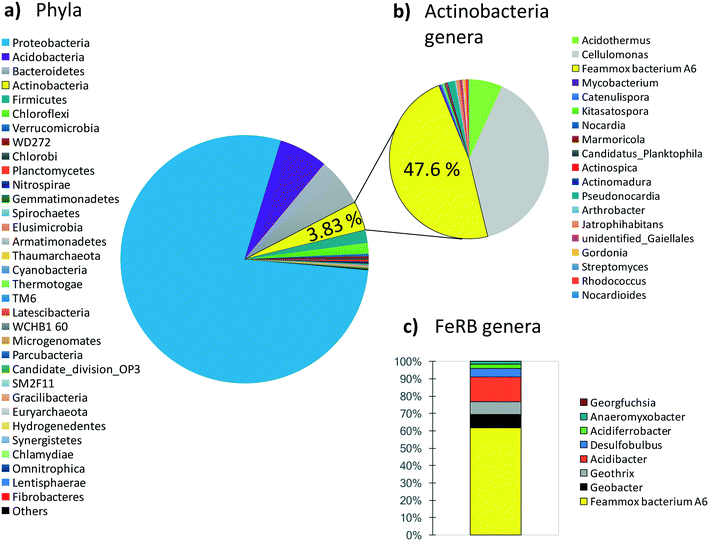
Since maintaining pure cultures in a biological reactor for extended time periods is a challenge, and since A6 might not remain active in pure cultures for long periods,7 we also tested the performance of an A6 enrichment culture in MECs. The goal was to determine if, as for the case of the pure culture, the enrichment culture could also produce current as a result of NH4+ oxidation. Results for the MECs with the A6 enrichment culture show that MECs produced an average current density (Id) of 2.5 A m−3 resulting in a CE of 5.4% and removed a total of 0.52 mM NH4+ after 3 weeks of operation (Fig. 6).The microbial community of a working MEC enrichment culture had a relative abundance of 3.83% Actinobacteria, the phylum to which A6 belongs to. This finding is consistent with the relative abundance of Actinobacteria found on electrodes deployed in the field or in constructed wetlands (2–5%).14 The relative abundance of A6 in these MECs is also similar to that obtained by Huang and Jaffé (2015)9 for an A6 enrichment culture in a membrane reactor to which ferrihydrite was provided as the electron acceptor. Within the Actinobacteria, at the genus level, 47.6% was classified as Feammox bacterium A6, i.e. Acidimicrobiaceae sp. A6, which had a relative abundance of 61.6% of the total diversity of all Fe-reducing bacteria present in the culture (Fig. 7). No other NH4+ oxidizing bacteria were detected at the genus level (for complete taxonomy of the microbial community composition of the top 100 most abundant genera from a MEC with an A6 enrichment culture see Table S2†).
Actinobacteria is a phylum frequently present in BES14 but normally is not considered in the microbial analysis of such systems, perhaps due to its lower abundance in comparison with more conspicuous groups such as Proteobacteria, which in the enrichment culture used here had a relative abundance of 78.3% of the total phyla. Proteobacteria is a major phylum that contains a great diversity of bacteria, comprising many widespread ones, including some of the most commonly present and extensively studied electrogenic microorganisms, namely, Geobacter spp. and Shewanella spp.11,12,48 However, regardless of the relative abundance of Proteobacteria in our system, Feammox bacteria A6 abundance made up 61.6% of all Fe-reducing bacteria (FeRB), all of the other genera of iron reducers are Proteobacteria except for Geothrix (Acidobacteria). The other FeRB that are also known to be electrogenic bacteria that can power BES11 include Geobacter (7.8%), Geothrix (7.2%), and Desulfobulbus (4.7%); however, they require organic C as their electron source49–51 and this was not provided to the MECs described here. The only source of C added was CO2 because A6 is an autotroph7 and we attribute the presence of other FeRB to remnants of the initial microbial community, and any carbon heterotrophic FeBR might be using would come from biomass turnover or any extracellular C produced by A6. Various bacteria, including iron reducers such as Geobacter sp.52 and A6 (ref. 7), can also use H2 as an electron donor, for this reason the headspace of MECs was flushed periodically, to avoid its interference in NH4+ removal and overall A6 activity and current production from other bacteria.
There are a limited number of studies on bioelectrochemical NH4+ oxidation using electrodes as the electron acceptor, and they conclude that the key organism responsible for this process is Nitrosomonas (Proteobacteria),33,34,45 a group not found in our MECs. Nitrosomonas are common aerobic nitrifiers present in soil, freshwater and wastewater. Qu et al. (2014)45 inoculated their system with freshwater sediments, and Zhan et al. (2014)33 and Vilajeliu-Pons et al. (2018)34 inoculated the anode chamber, of a dual-chamber bioelectrochemical system, with sludge from wastewater treatment plants. It is not surprising that they encountered nitrifiers in their systems; however, the mechanism used for anaerobic NH4+ oxidation by organisms that are normally aerobes, and how they produce current, are still unknown. It has been shown that commonly FeRB, such as Geobacter or Geothrix, have the ability to be electrogenic and thus transfer electrons to an anode, thus producing a current. A6 is an FeRB that carries out Feammox, the only fully anaerobic NH4+ oxidation process known, hence, we attribute the current production and ammonium oxidation in the MECs in part to A6 activity, especially since no other organism was present in the pure A6 culture MECs. No current was observed in the control MECs with dead A6, abiotic MECs or in the MEC operated with live A6 but without NH4+.
4. Conclusions
The results presented here demonstrate that the Feammox process, i.e. oxidation of ammonium by Acidimicrobiaceae sp. A6, can be carried out in bioelectrochemical reactors in the absence of ferric iron. A6 biomass can be sustained in equal concentrations in MEC reactors as in batch reactors containing Fe(III), and the relative numbers of A6 in MECs with an A6 enrichment culture were similar to those observed in a Feammox membrane reactor with ferrihydrite as the electron acceptor.Energy production is not the objective of oxidizing NH4+ in the MECs; the objective is rather to develop an energy efficient method for oxidizing NH4+ without the need for aeration and as an alternate method to Anammox, and the results presented here show that this is feasible and warrants further development. However, further R&D and process optimization is required for its commercial implementation and application for NH4+ or co-metabolic trace pollutant removal.
Conflicts of interest
There are no conflicts of interest.Acknowledgements
Funding for this research was provided by the Andlinger Center for Energy and the Environment and by the Princeton Environmental Institute. The Instituto de Fomento al Talento Humano – Ecuador and the Mary and Randall Hack'69 Research Grant provided funding for Melany Ruiz-Urigüen. We thank Dr. Bruce Logan for providing the first MEC reactors that were used as prototypes for the MECs used here and for his insightful ideas, Dr. Xiuping Zhu for her valuable advice on the MEC set-up, and Dr. Jason Ren and Dr. Lu Lu for their advice on MEC operation and this manuscript. We thank Dr. Chen Chen from the South China Environmental Institute for her collaboration on sequencing. From Princeton University, we thank Dr. Shan Huang for maintaining and providing A6 cultures and all members from the Steingart group for their assistance in the set-up of the electrochemical analysis. The authors acknowledge the use of Princeton's Imaging and Analysis Center, which is partially supported by the Princeton Center for Complex Materials, a National Science Foundation (NSF)-MRSEC program (DMR-1420541), and Florence Ling for her assistance in using the E-SEM.References
- P. Ciais and C. Sabine, IPCC Report. Chapter 6: Carbon and Other Biogeochemical Cycles, IPCC Report, Chapter 6: Carbon and Other Biogeochemical Cycles, 2013, pp. 465–570.
- S.-Y. Leu, D. Rosso, L. E. Larson and M. K. Stenstrom, Real-Time Aeration Efficiency Monitoring in the Activated Sludge Process and Methods to Reduce Energy Consumption and Operating Costs, Water Environ. Res., 2009, 81, 2471–2481 CrossRef CAS PubMed.
- D. Austin and J. Nivala, Energy requirements for nitrification and biological nitrogen removal in engineered wetlands, Ecol Eng, 2009, 35, 184–192 CrossRef.
- J. C. Clement, J. Shrestha, J. G. Ehrenfeld and P. R. Jaffé, Ammonium oxidation coupled to dissimilatory reduction of iron under anaerobic conditions in wetland soils, Soil Biol. Biochem., 2005, 37, 2323–2328 CrossRef CAS.
- S. Sawayama, Possibility of anoxic ferric ammonium oxidation, J. Biosci. Bioeng., 2006, 101, 70–72 CrossRef CAS PubMed.
- J. Shrestha, J. J. Rich, J. G. Ehrenfeld and P. R. Jaffé, Oxidation of Ammonium to Nitrite Under Iron-Reducing Conditions in Wetland Soils, Soil Sci., 2009, 174, 156–164 CrossRef CAS.
- S. Huang and P. R. Jaffé, Isolation and characterization of an ammonium-oxidizing iron reducer: Acidimicrobiaceae sp. A6, PLoS One, 2018, 13, e0194007 CrossRef PubMed.
- W. H. Yang, K. A. Weber and W. L. Silver, Nitrogen loss from soil through anaerobic ammonium oxidation coupled to iron reduction, Nat. Geosci., 2012, 5, 538–541 CrossRef CAS.
- S. Huang and P. R. Jaffé, Characterization of incubation experiments and development of an enrichment culture capable of ammonium oxidation under iron-reducing conditions, Biogeosciences, 2015, 12, 769–779 CrossRef.
- W. Shuai and R. P. Jaffé, Anaerobic ammonium oxidation coupled to iron reduction in constructed wetland mesocosms, Sci. Total Environ., 2019, 648, 984–992 CrossRef CAS PubMed.
- B. E. Logan, Exoelectrogenic bacteria that power microbial fuel cells, Nat. Rev. Microbiol., 2009, 7, 375–381 CrossRef CAS PubMed.
- D. R. Lovley, The microbe electric: conversion of organic matter to electricity, Curr. Opin. Biotechnol., 2008, 19, 564–571 CrossRef CAS PubMed.
- G. W. Zhou, X. R. Yang, H. Li, C. W. Marshall, B. X. Zheng, Y. Yan, J. Q. Su and Y. G. Zhu, Electron Shuttles Enhance Anaerobic Ammonium Oxidation Coupled to Iron(III) Reduction, Environ. Sci. Technol., 2016, 50, 9298–9307 CrossRef CAS PubMed.
- M. Ruiz-Urigüen, W. Shuai and P. R. Jaffé, Electrode Colonization by the Feammox Bacterium Acidimicrobiaceae sp. Strain A6, Appl. Environ. Microbiol., 2018, 84, e02029-18 CrossRef PubMed.
- J. Ge, S. Huang, I. Han and P. R. Jaffé, Degradation of tetra- and trichloroethylene under iron reducing conditions by Acidimicrobiaceae sp. A6, Environ. Pollut., 2019, 247, 248–255 CrossRef CAS PubMed.
- P. R. Jaffé and S. Huang, Defluorination of PFAS via Ammonium Oxidation under Iron Reducing Conditions, 2019 RemTEC Summit, 2019, https://www.remtecsummit.com/peter-jaffe.
- D. F. Call and B. E. Logan, A method for high throughput bioelectrochemical research based on small scale microbial electrolysis cells, Biosens. Bioelectron., 2011, 26, 4526–4531 CrossRef CAS PubMed.
- B. E. Logan, B. Hamelers, R. Rozendal, U. Schroder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete and K. Rabaey, Microbial fuel cells: methodology and technology, Environ. Sci. Technol., 2006, 40, 5181–5192 CrossRef CAS PubMed.
- U. Schwertmann and R. M. Cornell, Iron Oxides in the Laboratory: Preparation and Characterization, Wiley-VCH, 2000 Search PubMed.
- L. L. Stookey, Ferrozine- A New Spectrophotometric Reagent for Iron, Anal. Chem., 1970, 42, 779–781 CrossRef CAS.
- J. Komlos and P. R. Jaffé, Effect of iron bioavailability on dissolved hydrogen concentrations during microbial iron reduction, Biodegradation, 2004, 15, 315–325 CrossRef CAS PubMed.
- J. G. Caporaso, C. L. Lauber, W. A. Walters, D. Berg-Lyons, C. A. Lozupone, P. J. Turnbaugh, N. Fierer and R. Knight, Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 4516–4522 CrossRef CAS PubMed.
- T. Magoc and S. L. Salzberg, FLASH: fast length adjustment of short reads to improve genome assemblies, Bioinformatics, 2011, 27, 2957–2963 CrossRef CAS PubMed.
- J. G. Caporaso, J. Kuczynski, J. Stombaugh, K. Bittinger, F. D. Bushman, E. K. Costello, N. Fierer, A. G. Pena, J. K. Goodrich, J. I. Gordon, G. A. Huttley, S. T. Kelley, D. Knights, J. E. Koenig, R. E. Ley, C. A. Lozupone, D. McDonald, B. D. Muegge, M. Pirrung, J. Reeder, J. R. Sevinsky, P. J. Turnbaugh, W. A. Walters, J. Widmann, T. Yatsunenko, J. Zaneveld and R. Knight, QIIME allows analysis of high-throughput community sequencing data, Nat. Methods, 2010, 7, 335–336 CrossRef CAS PubMed.
- R. C. Edgar, B. J. Haas, J. C. Clemente, C. Quince and R. Knight, UCHIME improves sensitivity and speed of chimera detection, Bioinformatics, 2011, 27, 2194–2200 CrossRef CAS PubMed.
- R. C. Edgar, UPARSE: highly accurate OTU sequences from microbial amplicon reads, Nat. Methods, 2013, 10, 996–998 CrossRef CAS PubMed.
- D. R. Lovley, J. D. Coates, E. L. Blunt-Harris, E. J. P. Phillips and J. C. Woodward, Humic substances as electron acceptors for microbial respiration, Nature, 1996, 382, 445–448 CrossRef CAS.
- E. E. Rios-Del Toro, E. I. Valenzuela, J. E. Ramírez, N. E. López-Lozano and F. J. Cervantes, Anaerobic Ammonium Oxidation Linked to Microbial Reduction of Natural Organic Matter in Marine Sediments, Environ. Sci. Technol. Lett., 2018, 5, 571–577 CrossRef CAS.
- Z. M. Summers, H. E. Fogarty, C. Leang, A. E. Franks, N. S. Malvankar and D. R. Lovley, Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria, Science, 2010, 330, 1413–1415 CrossRef CAS PubMed.
- S. Orsetti, C. Laskov and S. B. Haderlein, Electron transfer between iron minerals and quinones: estimating the reduction potential of the Fe(II)-goethite surface from AQDS speciation, Environ. Sci. Technol., 2013, 47, 14161–14168 CrossRef CAS PubMed.
- D. A. Lowy, L. M. Tender, J. G. Zeikus, D. H. Park and D. R. Lovley, Harvesting energy from the marine sediment-water interface II-Kinetic activity of anode materials, Biosens. Bioelectron., 2006, 21, 2058–2063 CrossRef CAS PubMed.
- C. H. Feng, L. Ma, F. B. Li, H. J. Mai, X. M. Lang and S. S. Fan, A polypyrrole/anthraquinone-2,6-disulphonic disodium salt (PPy/AQDS)-modified anode to improve performance of microbial fuel cells, Biosens. Bioelectron., 2010, 25, 1516–1520 CrossRef CAS PubMed.
- G. Zhan, L. Zhang, Y. Tao, Y. Wang, X. Zhu and D. Li, Anodic ammonia oxidation to nitrogen gas catalyzed by mixed biofilms in bioelectrochemical systems, Electrochim. Acta, 2014, 135, 345–350 CrossRef CAS.
- A. Vilajeliu-Pons, C. Koch, M. D. Balaguer, J. Colprim, F. Harnisch and S. Puig, Microbial electricity driven anoxic ammonium removal, Water Res., 2018, 130, 168–175 CrossRef CAS PubMed.
- X. P. Zhu, M. D. Yates and B. E. Logan, Set potential regulation reveals additional oxidation peaks of Geobacter sulfurreducens anodic biofilms, Electrochem. Commun., 2012, 22, 116–119 CrossRef CAS.
- X. Zhu, M. D. Yates, M. C. Hatzell, H. Ananda Rao, P. E. Saikaly and B. E. Logan, Microbial community composition is unaffected by anode potential, Environ. Sci. Technol., 2014, 48, 1352–1358 CrossRef CAS PubMed.
- A. Eftekhari, Electrocatalysts for hydrogen evolution reaction, Int. J. Hydrogen Energy, 2017, 42, 11053–11077 CrossRef CAS.
- A. Escapa, M. I. San-Martin, R. Mateos and A. Moran, Scaling-up of membraneless microbial electrolysis cells (MECs) for domestic wastewater treatment: Bottlenecks and limitations, Bioresour. Technol., 2015, 180, 72–78 CrossRef CAS PubMed.
- J. Z. Zhou, W. Z. Liu, Y. Deng, Y. H. Jiang, K. Xue, Z. L. He, J. D. Van Nostrand, L. Y. Wu, Y. F. Yang and A. J. Wang, Stochastic Assembly Leads to Alternative Communities with Distinct Functions in a Bioreactor Microbial Community, mBio, 2013, 4, 8 Search PubMed.
- N. Klueglein and A. Kappler, Abiotic oxidation of Fe(II) by reactive nitrogen species in cultures of the nitrate-reducing Fe(II) oxidizer Acidovorax sp. BoFeN1 - questioning the existence of enzymatic Fe(II) oxidation (vol 11, pg 180, 2013), Geobiology, 2013, 11, 396 CrossRef CAS PubMed.
- N. Klueglein, F. Zeitvogel, Y. D. Stierhof, M. Floetenmeyer, K. O. Konhauser, A. Kappler and M. Obst, Potential Role of Nitrite for Abiotic Fe(II) Oxidation and Cell Encrustation during Nitrate Reduction by Denitrifying Bacteria, Appl. Environ. Microbiol., 2014, 80, 1051–1061 CrossRef PubMed.
- L. Lu and Z. J. Ren, Microbial electrolysis cells for waste biorefinery: A state of the art review, Bioresour. Technol., 2016, 215, 254–264 CrossRef CAS PubMed.
- J. Ditzig, H. Liu and B. E. Logan, Production of hydrogen from domestic wastewater using a bioelectrochemically assisted microbial reactor (BEAMR), Int. J. Hydrogen Energy, 2007, 32, 2296–2304 CrossRef CAS.
- H. Liu, H. Hu, J. Chignell and Y. Fan, Microbial electrolysis: novel technology for hydrogen production from biomass, Biofuels, 2010, 1, 129–142 CrossRef CAS.
- B. Qu, B. Fan, S. Zhu and Y. Zheng, Anaerobic ammonium oxidation with an anode as the electron acceptor, Environ. Microbiol. Rep., 2014, 6, 100–105 CrossRef CAS PubMed.
- B. Yang, L. Hoober-Burkhardt, F. Wang, G. K. S. Prakash and S. R. Narayanan, An Inexpensive Aqueous Flow Battery for Large-Scale Electrical Energy Storage Based on Water-Soluble Organic Redox Couples, J. Electrochem. Soc., 2014, 161, A1371–A1380 CrossRef CAS.
- A. E. Franks, N. Malvankar and K. P. Nevin, Bacterial biofilms: the powerhouse of a microbial fuel cell, Biofuels, 2010, 1, 589–604 CrossRef CAS.
- K. H. Williams, K. P. Nevin, A. Franks, A. Englert, P. E. Long and D. R. Lovley, Electrode-based approach for monitoring in situ microbial activity during subsurface bioremediation, Environ. Sci. Technol., 2010, 44, 47–54 CrossRef CAS PubMed.
- D. R. Bond and D. R. Lovley, Electricity Production by Geobacter sulfurreducens Attached to Electrodes, Appl. Environ. Microbiol., 2003, 69, 1548–1555 CrossRef CAS PubMed.
- D. R. Bond and D. R. Lovley, Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans, Appl. Environ. Microbiol., 2005, 71, 2186–2189 CrossRef CAS PubMed.
- D. E. Holmes, D. R. Bond and D. R. Lovley, Electron Transfer by Desulfobulbus propionicus to Fe(III) and Graphite Electrodes, Appl. Environ. Microbiol., 2004, 70, 1234–1237 CrossRef CAS PubMed.
- F. Caccavo, Jr., D. J. Lonergan, D. R. Lovley, M. Davis, J. F. Stolz and M. J. McInerney, Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism, Appl. Environ. Microbiol., 1994, 60, 3752–3759 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ew00366e |
| This journal is © The Royal Society of Chemistry 2019 |