Complex organic particulate artificial sewage (COPAS) as surrogate wastewater in anaerobic assays
Abstract
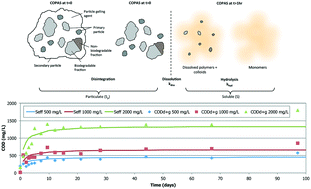
Storage, preservation, and batch-to-batch variability of the influent composition are challenges to laboratory-scale research in the wastewater treatment field. Synthetic wastewaters are commonly used, but many fail to capture the complexity of actual wastewater, especially in terms of particulate organic matter. An alternative synthetic sewage, referred to as complex organic particulate artificial sewage (COPAS), is introduced in this study. COPAS is easily prepared and is based on a simple recipe that uses granular dried cat food as the source of particulate organic matter. On a weight basis, COPAS particles consist of proteins (40%), fats (17%), carbohydrates (43%), and trace nutrients, including vitamins and trace metals. Dissolution/hydrolysis batch tests of COPAS particles indicate that the dissolved organic carbon and nitrogen are released rapidly within the first two hours (approximately 10% C and 17% N). The remaining fraction of organic C and N remain in the particulate form for further dissolution and/or biodegradation. A khyd of 0.82 d−1 was calculated for COPAS based on its protein, fat, and carbohydrate contents. Further, anaerobic bioassays prove the biodegradability of COPAS with approximately 60% theoretical methane produced after 45 days of incubation.
- This article is part of the themed collection: Best Papers 2019 – Environmental Science: Water Research & Technology


 Please wait while we load your content...
Please wait while we load your content...