Open Access Article
Open Access ArticleStep-by-step analysis of drinking water treatment trains using size-exclusion chromatography to fingerprint and track protein-like and humic/fulvic-like fractions of dissolved organic matter†
Alexey
Ignatev
 * and
Tuula
Tuhkanen
* and
Tuula
Tuhkanen
Department of Biological and Environmental Science, University of Jyväskylä, PO Box 35, FI-40014, Jyväskylä, Finland. E-mail: alexey.n.ignatev@jyu.fi; alexignat@gmail.com; tuula.a.tuhkanen@jyu.fi
First published on 4th July 2019
Abstract
This paper provides a glimpse into the removal of dissolved organic matter (DOM) during conventional drinking water treatment and evaluates the potential of high-performance size-exclusion chromatography (HPSEC) as a supplementary tool for routine monitoring of drinking water treatment plants (DWTPs). Two DWTPs in Central Finland were systematically evaluated using HPSEC with simultaneous UV and fluorescence detection. For tyrosine-like, tryptophan-like, and humic/fulvic-like DOM fractions of various molecular weight (MW) values, the total and step-by-step removal efficiencies were estimated along the treatment trains. Overall, both DWTPs removed ∼70% of dissolved organic carbon (DOC) and reduced by 80–90% the total fluorescence and total UV absorbance (UVA). DOM fractions of high MW > 1500 Da were efficiently >95% removed. Fractions of intermediate MW 750–1500 Da were 80–90% removed, whereas the removal efficiency for fractions of low MW < 600 Da was in the range of 60–70%. The lowest removal efficiency across all fractions and detection was observed by UVA210 for the DOM fraction of small MW < 300 Da, for which only 20–30% was removed. In one of the DWTPs, the chromatographic area of this fraction occasionally increased, indicating the formation of degradation and/or oxidation products. Pre-ozonation of raw water reduced total tyrosine- and tryptophan-like fluorescence by ∼30%, humic/fulvic-like fluorescence by ∼20%, and total UVA254 by ∼25%. In the conventional coagulation/flocculation, high MW fractions were removed almost completely, whereas the removal of low MW fractions was only ∼20%. The coagulability of individual fractions was correlated with their hydrophobicity/hydrophilicity estimated using the ratio of UVA210/UVA254. In one of the DWTPs, oxidation with ClO2 induced the formation of DOM with MW 750–1500 Da due to the polymerization or release of DOM from colloidal matter. This new DOM was partly removed in the subsequent sand and activated carbon (AC) filtration and partly ended up in the treated water. In the AC filters, 20–60% of DOM fractions of low MW < 600 Da were removed, and fluorescent compounds exhibited two-fold higher removal efficiencies compared to UV absorbing compounds. Analyses of SUVA and the ratio of UVA210/UVA254 provided surrogate quantification of the aromatic character and hydrophobic/hydrophilic properties of unfractionated and fractionated DOM.
Water impactThis is one the first studies where size-exclusion chromatography coupled with both UV and fluorescence detection is evaluated as a routine tool for advanced monitoring of the removal of dissolved organic matter in conventional drinking water treatment plants. To determine what is removed and what is not removed, protein-like compounds and humic substances were monitored step-by-step and fraction-by-fraction. |
1. Introduction
Accelerating anthropogenic activity, climate change, and other megatrends lead to deterioration of water quality globally through chemical contamination, eutrophication, algal bloom, etc.1 Particularly, soil acidification, intensifying rain and drought events are causing a rapid increase of dissolved organic matter (DOM) content in lakes and rivers.2 For example, the dissolved organic carbon (DOC) concentration in Lake Päijänne, which is the second largest lake in Finland, increased from ∼5.5 to ∼7.5 mg C per L between 2000 and 2015.3The quality and quantity of DOM in raw water sources influence the choice of appropriate treatment methods and determine the demands of coagulants, oxidants, and disinfectants in drinking water treatment plants (DWTPs). Efficient engineering design and operational control are further challenged by possible seasonal variations of DOM composition.4 Unremoved DOM can cause adverse effects, such as formation of toxic and mutagenic disinfection by-products (DBPs) upon chlorination,5,6 microbial growth in distribution networks,7 and unpleasant color, taste, and odor of tap water.8 To prevent these problems, routine monitoring of DOM quality and quantity is needed in all the steps from the source to the tap.
DOM in surface water is a highly complex system of humic and fulvic compounds, lignins, carbohydrates, proteins, and other heterogeneous components of not only mostly natural, but also anthropogenic origin. Thus, a comprehensive characterization of DOM requires a multi-method analytical approach.9
High performance size-exclusion chromatography (HPSEC) is an established technique to evaluate the removal of DOM along drinking water treatment trains.10 Relatively fast, easy and affordable HPSEC analysis requires small sample volume and minimal pre-treatment.11 In HPSEC, molecules are separated according to their apparent molecular weights (MWs) into fractions, which can be simultaneously characterized by various detectors. Typically, UV and online DOC detectors are used. While DOC detection is universal, it does not provide insights into the chemistry of individual fractions. Monitoring UV absorbance at 254 nm (UVA254) allows robust detection of conjugated double bonds and aromatic structures. At the same time, UVA254 detection cannot discriminate different classes of aromatic compounds, such as humic substances and aromatic proteins. Sometimes wavelengths other than 254 nm are explored. For example, a correlation between UVA210 and microbiological water quality parameters was reported.12
Humic substances, which comprise 40–80% of raw water DOC,13,14 have featureless UV-vis spectra that provide scarce information.15 Moreover, a rather low sensitivity of UV detection limits the applicability of HPSEC-UV for analysis of samples with low DOC content, such as drinking water. Thus, additional detectors are necessary to obtain more information about DOM fractions.16
The high sensitivity and selectivity of fluorescence detection allow determination of various organic compounds at low concentrations, particularly, in drinking water.17 Besides humic/fulvic-like fluorescent compounds, a diverse pool of protein-like compounds can be monitored by their characteristic tyrosine- and tryptophan-like fluorescence. Recently, tryptophan-like fluorescence was suggested as a surrogate measure of microbial contamination risk.18 Usually, excitation–emission matrix (EEM) fluorescence spectroscopy with parallel factor analysis (PARAFAC) is suggested to characterize the removal of fluorescent DOM.19,20 At the same time, the combination of HPSEC fractionation and fluorescence detection is overlooked in the field of water analysis and technology. To the best of our knowledge, only a few studies have applied HPSEC-fluorescence to study drinking water treatment21,22 and none of them undertook systematic comparative characterization of DOM fractions at different excitation/emission wavelengths (λex/λem).
Thus, we aimed to evaluate HPSEC-UV-fluorescence as a supplementary tool for routine (and, potentially, online and on-site) monitoring of DOM along drinking water treatment trains. For this, samples of raw, processed, and treated water were collected from two conventional DWTPs and systematically analyzed to identify removable and refractory DOM fractions and to assess the treatment performance – overall, step-by-step, and fraction-by-fraction.
2. Materials and methods
2.1. Design of DWTPs
Principal schemes of the DWTPs assessed in this study are given in Fig. 1.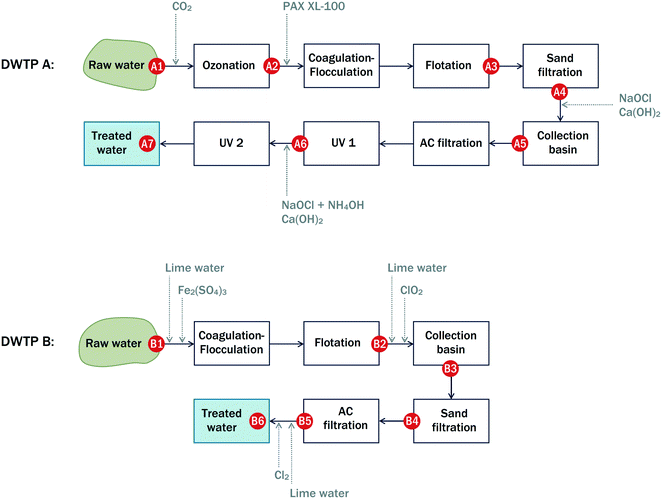 | ||
| Fig. 1 Principal schemes of DWTPS A and B and indicative locations of sampling points A1–A7 and B1–B6. | ||
DWTP A (city of Jyväskylä, Finland) pumps raw water from the nearby Lake Tuomiojärvi and has an average water flow of ∼300 m3 h−1. The DWTP employs pre-ozonation to oxidize manganese present in the raw water. The ozonation is followed by coagulation–flocculation (with the addition of polyaluminum coagulant KEMIRA PAX-XL 100), flotation combined with sand filtration, intermediate oxidation with NaOCl, activated carbon (AC) filtration (eight parallel AC tanks), intermediate low-power UV treatment, post-oxidation, and final high-power UV treatment.
DWTP B (city of Tampere, Finland) pumps raw water from Lake Roine, located at a distance of 7 km from the DWTP, and has an average water flow of ∼1700 m3 h−1. Conventional coagulation–flocculation (with the addition of ferric sulphate) is followed by flotation, intermediate oxidation with ClO2, sand filtration, AC filtration, and final post-chlorination with Cl2. Alkalinization with lime is used to adjust hardness and alkalinity. Over a decade ago, the performance of DWTP B was studied using HPSEC-UV.23–25
2.2. Sampling and sample preparation
Each DWTP was sampled four times in total: DWTP A – in 2017 (September, October, and November) and 2018 (September), DWTP B – in 2017 (October) and 2018 (March, June, and August). Locations of the sampling points are indicated in Fig. 1.Water samples were collected into polypropylene bottles (pre-washed and rinsed three times with ultrapure water) and transported in cool boxes. Upon delivery to the laboratory, the samples were filtered through pre-washed 0.45 μm syringe filters (cellulose acetate from VWR, USA, or regenerated cellulose from Phenomenex, USA). HPSEC analyses were completed within a few days. Filtered aliquots for DOC analysis were frozen at −20 °C in polypropylene screw cap tubes (Sarstedt, Germany) and analyzed within several weeks.
2.3. HPSEC method
All the samples were analyzed using an HPLC Shimadzu LC-30AD equipped with online degassing units Shimadzu DGU-20A5R and DGU-20A3R, a column oven Shimadzu CTO-20AC, an autosampler Shimadzu SIL-30AC, a photodiode array (PDA) detector Shimadzu SPD-M20A, and a fluorescence detector Shimadzu RF-20A XS. The separation column was a silica-based Yarra SEC-3000 (300 × 7.6 mm, Phenomenex, USA).The eluent was 5 mmol L−1 phosphate buffer (pH 6.8, ionic strength 10 mmol L−1) with β(Na2HPO4·2H2O) = 0.45 g L−1 and β(NaH2PO4·2H2O) = 0.39 g L−1 at a flow rate of 1 mL min−1. The eluent composition was chosen based on previous studies11,23,26,27 to achieve high chromatographic resolution. Analytical grade Na2HPO4·2H2O and NaH2PO4·2H2O were purchased from VWR, Belgium, and Merck, Germany, respectively. Ultrapure water was generated using an “Ultra Clear UV plus TM” system (SG Water, Germany). The eluent was pre-filtered through 0.2 μm cellulose acetate membrane filters (Whatman, Germany).
The main parameters of the HPSEC method are summarized in Table S1.† The monitored λex/λem values were chosen according to typical EEM fluorescence spectra of raw water (Fig. S1†) to represent tyrosine-like (region B1: 220/310 nm), tryptophan-like (region T2: 270/355 nm), and humic/fulvic-like (region C1: 330/425 nm and region C2: 390/500 nm) fluorescent compounds. Region A of humic/fulvic-like fluorescence and region T1 of tryptophan-like fluorescence were not monitored to avoid possible inner filter effects due to intense UV absorption below 250 nm and to minimize the total number of HPSEC analyses. Region B1 of tyrosine-like fluorescence was chosen due to the lower noise and higher intensity of tyrosine-like fluorescence at the shorter excitation wavelengths compared to those at the longer excitation wavelengths of region B2 (Fig. S2†).
The void volume, determined with blue dextran (Sigma-Aldrich, Sweden), was ∼5.4 mL (elution time ∼5.4 min), and the permeation volume, determined with acetone, was ∼12.2 mL (elution time ∼12.2 min). The size-exclusion column was calibrated with polystyrene sulfonate (PSS) standards of 210, 1600, 3200, 4800, 6400, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 32
000, and 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da (Sigma-Aldrich, Germany). The calibration curve is shown in Fig. S3.†
000 Da (Sigma-Aldrich, Germany). The calibration curve is shown in Fig. S3.†
Recently, the same HPSEC-UV-fluorescence method was used to characterize treated and untreated wastewater and to evaluate the efficiency of wastewater treatment.11
2.4. Processing of HPSEC data
Raw chromatographic data were exported from proprietary software Shimadzu LabSolutions LC/GC Version 5.51 to ASCII text files and processed in MathWorks MATLAB R2016b. In-house scripts were used to automatically correct baseline, recognize individual fractions, and integrate each chromatogram.Total fluorescence and total UVA were obtained by integrating the HPSEC chromatograms in the elution time range of 4.5–15.0 min to combine all the peaks eluted within fractions I–VI. Thus, total fluorescence and total UVA represent the properties of unfractionated (whole) water samples.
Removal efficiencies were calculated for DOM fractions I–VI and for whole water for each treatment step according to the following equation
| Removal efficiency = (1 − Aeffluent/Ainfluent) × 100% | (1) |
The SUVA index of the whole water samples was calculated as follows:
| SUVA = (total UVA254/DOC) × (u/Vinj) × 100 | (2) |
The weight average MW (MW), number average MW (MN), and polydispersity (ρ) were calculated from the HPSEC-UV and HPSEC-fluorescence chromatograms according to (3)–(5).28
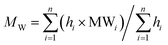 | (3) |
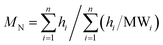 | (4) |
| ρ = MW/MN | (5) |
2.5. DOC analysis
A total organic carbon analyzer Shimadzu TOC-L with an autosampler ASI-L was used to determine the DOC content. The non-purgeable organic carbon method was selected. The analyzer was calibrated in the DOC range of 0–30 mg C per L with standard solutions of potassium phthalate prepared by automatic dilution of a fresh stock. Prior to analysis, vials were calcined at 400 °C for 4 h in air. All the samples were acidified to pH < 2 with HCl and purged with N2 to strip dissolved inorganic carbon. The injection volume was 100 μL. The number or replicate injections (2 or 3) was chosen automatically using the analyzer.3. Results and discussion
3.1. Characterization of raw water DOM
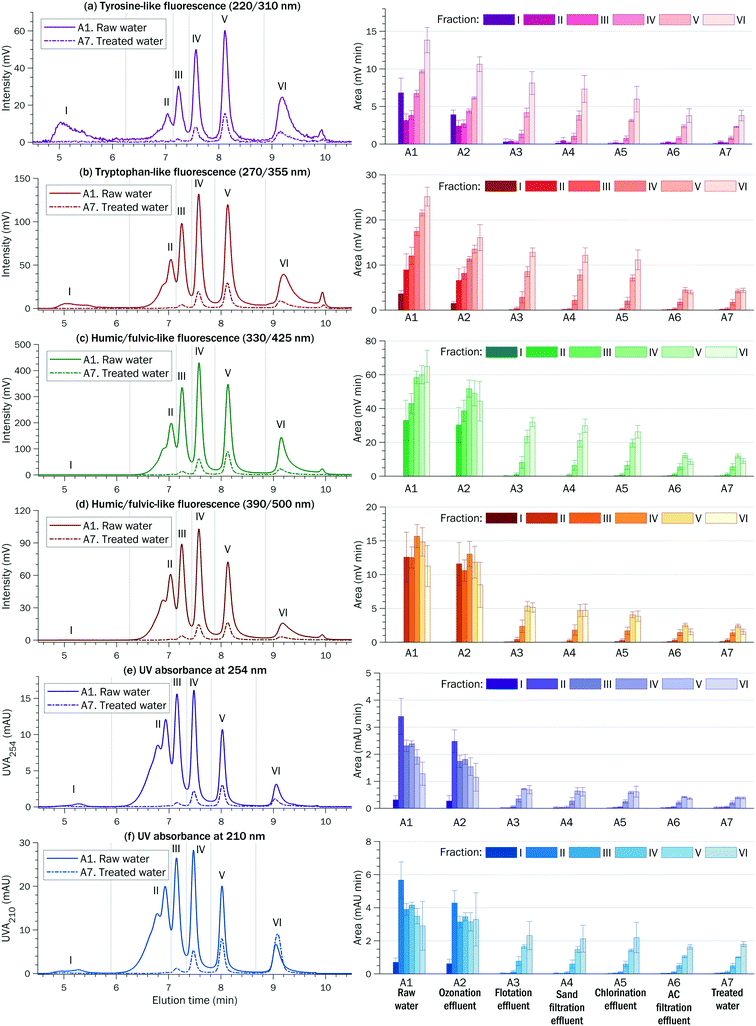
The SUVA of the raw water was in the range of 2–4 at both DWTPs (Tables 1 and S4†) indicating the complex DOM composition of humic and non-humic and hydrophobic and hydrophilic fractions of various MWs.29 Depending on the detector (fluorescence or UV), six to eight peaks were observed in the HPSEC chromatograms of raw water, which is typical for Finnish surface waters.26,30 These peaks were deliberately combined into six fractions as shown in Fig. 2 for DWTP A and in Fig. S4† for DWTP B. MW decreases from fraction I to fraction VI.
| A1 | A2 | A3 | A4 | A5 | A6 | A7 | |
|---|---|---|---|---|---|---|---|
| Raw water | Ozonation effluent | Flotation effluent | Sand filtration effluent | Chlorination effluent | AC filtration effluent | Treated water | |
| DOC (mg C per L) | 6.9 ± 0.6 | 6.5 ± 0.3 | 3.1 ± 0.4 | 2.5 ± 0.4 | 2.7 ± 0.4 | 2.3 ± 0.4 | 2.0 ± 0.2 |
| SUVA (L mg−1 m−1) | 3.4 ± 0.4 | 2.8 ± 0.4 | 1.2 ± 0.2 | 1.3 ± 0.4 | 1.1 ± 0.2 | 0.9 ± 0.1 | 1.1 ± 0.3 |
| UVA210/UVA254 | 1.8 ± 0.1 | 2.0 ± 0.1 | 2.6 ± 0.3 | 2.7 ± 0.4 | 2.8 ± 0.2 | 3.2 ± 0.1 | 3.3 ± 0.4 |
| Total UVA (mAU min) | |||||||
| 254 nm | 11.6 ± 1.1 | 9.0 ± 1.4 | 1.8 ± 0.1 | 1.6 ± 0.5 | 1.5 ± 0.2 | 1.1 ± 0.1 | 1.1 ± 0.2 |
| 210 nm | 20.8 ± 2.7 | 17.9 ± 2.9 | 4.9 ± 0.7 | 4.3 ± 1.0 | 4.4 ± 0.9 | 3.3 ± 0.2 | 3.5 ± 0.3 |
| Total fluorescence (mV min) | |||||||
| Tyrosine-like (220/310 nm) | 44.0 ± 1.6 | 30.2 ± 2.0 | 14.6 ± 2.7 | 13.0 ± 2.7 | 10.3 ± 1.2 | 7.5 ± 0.5 | 7.4 ± 0.5 |
| Tryptophan-like (270/355 nm) | 88.8 ± 7.1 | 57.3 ± 5.3 | 24.7 ± 2.4 | 22.5 ± 3.2 | 20.6 ± 2.3 | 10.8 ± 1.4 | 10.9 ± 1.5 |
| Humic/fulvic-like (330/425 nm) | 259.3 ± 21.4 | 214.4 ± 25.3 | 65.1 ± 6.8 | 58.4 ± 8.5 | 53.5 ± 6.2 | 27.9 ± 3.3 | 28.2 ± 3.1 |
| Humic/fulvic-like (390/500 nm) | 66.9 ± 8.8 | 55.6 ± 8.8 | 13.4 ± 1.8 | 11.6 ± 2.2 | 10.0 ± 1.3 | 6.0 ± 0.8 | 5.9 ± 0.7 |
Fraction I, eluted around the void volume, contributed to ∼15% of the total tyrosine-like and ∼5% of the total tryptophan-like fluorescence of the whole raw water samples. It is thought that this fraction consists of organic colloids (MW ≥ 50 kDa) and biopolymers (MW ≥ 10 kDa), such as polysaccharides, with some contribution from proteinic matter and amino sugars.2,31,32 Some researchers suggest that fraction I contains aggregates from self-association of humic substances.26,33 However, in this study, fraction I did not exhibit characteristic humic/fulvic-like fluorescence.
The MWs of DOM fractions II–VI were estimated using the calibration curve obtained with PSS standards (Fig. S3†). The PSS standards are considered similar to humic substances in terms of charge density and behavior in size-exclusion columns. However, due to debatable structural similarity and various secondary interactions, MWs estimated using PSS calibration should be considered apparent and indicative rather than true.34,35
Humic substances of high MW 1500–3200 Da were eluted in fraction II. Humic substances of low MW 750–1500 Da were eluted in fractions III and IV. Building blocks (breakdown products of humic substances) had a MW of 500–600 Da and were eluted in fraction V.
Fractions II–VI simultaneously exhibited tyrosine-like, tryptophan-like, and humic/fulvic-like fluorescence, suggesting that protein-like compounds and humic/fulvic-like substances were eluted together. However, HPSEC analysis alone does not allow determination of whether the observed fluorophores are present side-by-side in the same molecules or belong to different molecules that co-elute due to similar MWs or intermolecular association. Moreover, it is known that humic structures can incorporate protein-like fractions as a result of weak interactions based on π–π and/or van der Waals forces between the DOM components.36,37 Recent studies indicate that not only proteins but also humic supramolecules containing certain structures (derived from phenol or aniline) can contribute to protein-like fluorescence.38
“Red-shifted” humic/fulvic-like fluorescence at 390/500 nm (emission at longer wavelengths) can be related to highly conjugated aromatic compounds of high MW, whereas “blue-shifted” humic/fulvic-like fluorescence at 330/425 nm (emission at shorter wavelengths) can be assigned to compounds with lower aromaticity and lower MWs.39 Thus, fluorescence at 390/500 nm may be indicative of humic-like compounds, whereas fluorescence at 330/425 nm may be indicative of fulvic-like compounds. However, the same fluorophores are present in both humic and fulvic compounds and a clear differentiation between them based on fluorescence properties is not always possible.40
Tyrosine- and tryptophan-like fluorescence of raw water is attributed to amino acids, free, bound to proteins, or associated with high MW organic compounds, such as humic substances.39 Tyrosine residues in proteins and peptides do not emit fluorescence in the presence of tryptophan, because the emission energy of tyrosine residues is transferred to the excitation energy of the neighboring tryptophan residues.41 Thus, tyrosine-like fluorescence may indicate more degraded peptide materials, while tryptophan-like fluorescence may represent intact proteins and less degraded peptide materials.39
Fraction VI with apparent MW < 300 Da represented a diverse pool of low MW acids and neutrals (carboxylic acids, amino acids, sugars, purines, aldehydes, ketones, etc.).31,42
The humic/fulvic-like fluorescence of the raw water was evenly distributed across fractions II–VI, whereas tryptophan-like and tyrosine-like fluorescence was more pronounced in fractions IV–VI with lower MW. These fractions accounted for ∼70% of the total tyrosine-like and total tryptophan-like fluorescence in the raw water (Fig. 3 for DWTP A and Fig. S5† for DWTP B).
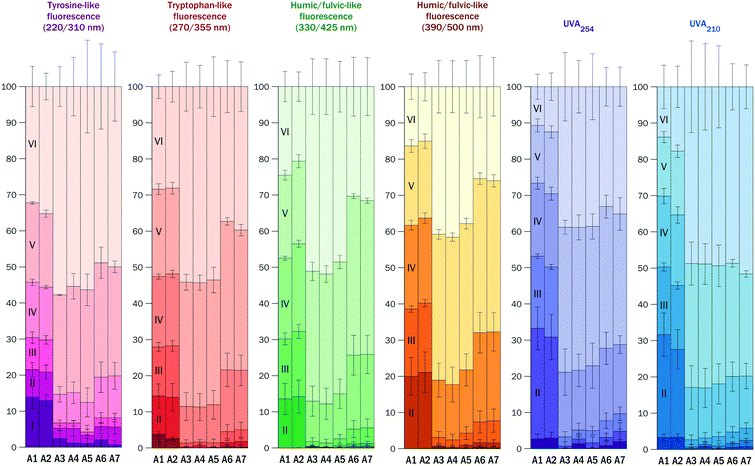 | ||
| Fig. 3 Relative abundance of fluorescent and UV absorbing DOM fractions I–VI in raw water (A1), processed water (A2–A6), and treated water (A7) at DWTP A (mean ± sd, n = 4). Locations of sampling points A1–A7 are shown in Fig.1. | ||
Calculated from the HPSEC-UV chromatograms, MW was ∼1500 Da and MN was ∼800 Da for both raw water samples at DWTPs A and B. These are similar to the values reported for several drinking water sources in Norway and Australia,2 but considerably smaller than the MW of 2114 Da and MN of 1385 Da reported for reference Suwannee River fulvic acid28 and the MW of 6102 Da and MN of 3873 Da determined for Nordic reference fulvic acid.45 These discrepancies could stem from the different eluents, size-exclusion standards and columns used in the studies.
The polydispersity (ρ) of the raw water DOM was ∼1.8 regardless of detection. ρ ∼ 1 indicates that the DOM is made up of compounds with similar MWs, while greater ρ indicates more complex mixtures of heterogeneous organic compounds.46
3.2. Overall DWTP performance
At both DWTPs, raw water and treated water are routinely sampled and analyzed by accredited laboratories, as required by operational protocols and regulations. Selected general water quality parameters are summarized in Tables S2 and S3† to complement data obtained in this study.Treatment efficiency was evaluated in terms of DOC removal, the decrease of UVA210 and UVA254, reduction of fluorescence signals at different λex/λem, changes of SUVA and the ratio of UVA210/UVA254 for whole water samples collected along the treatment trains (Table 1 for DWTP A and Table S4† for DWTP B).
Both DWTPs reduced the DOC content by ∼70%. The total UVA254 and total humic/fulvic-like fluorescence were reduced by ∼90% at DWTP A and by ∼85% at DWTP B. The total tyrosine- and tryptophan-like fluorescence signals were reduced by ∼86% at DWTP A and by ∼77% at DWTP B. The lowest removal efficiency was observed for the total UVA210: ∼83% reduction at DWTP A and ∼68% reduction at DWTP B. Aromatic and many aliphatic compounds as well as some inorganic ions (for example, nitrate anion) absorb at 210 nm and thus, UVA210 cannot be attributed to a particular category of compounds.
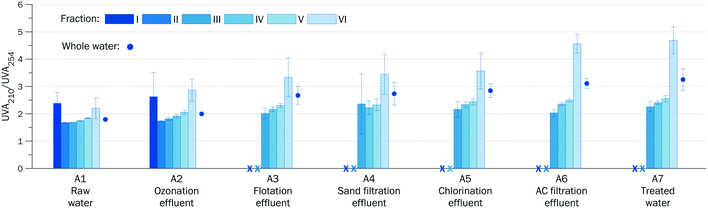
The decreasing SUVA and increasing ratio of UVA210/UVA254 (Tables 1 and S4†) indicate that the residual DOM in the treated water samples had a significantly lower aromatic character compared to the raw water samples. For surface raw water, higher SUVA corresponds to higher aromaticity, higher hydrophobicity and higher humic/fulvic content.
The ratio of UVA210/UVA254 was suggested as a surrogate indicator of the relative density of aliphatic functional groups and conjugated double bonds.47 Aliphatic functional groups typically have higher UVA210 and lower UVA254 whereas conjugated double bonds and aromatic structures have high UVA210 and high UVA254. Thus, the ratio of UVA210/UVA254 decreases with the increase in aromatic character. For example, the UVA210/UVA254 values of Suwannee River standard humic and fulvic acids are correspondingly 1.59 and 1.88,48 while for proteins of bovine serum albumin with low aromaticity the ratio is 13.50.49 The ratio of UVA210/UVA254 of fractions II–V indicates the fulvic character of the raw water DOM (Fig. 5 and S7†).
The HPSEC separation eliminated the inorganic component from all the fractions except fraction VI where inorganic ions, which can be UV absorbing, co-eluted with low MW DOM components. Due to this possible interference, the ratio of UVA210/UVA254 may not reflect the properties of low MW organic compounds in fraction VI.
The specific (DOC normalized) fluorescence decreased during the treatment by ∼45% for tyrosine-like and by ∼60% for tryptophan-like fluorescence, which are lower than the ∼70% decrease of SUVA and the specific humic/fulvic-like fluorescence at λex/λem of 390/500 nm. This indicates that protein-like DOM components are harder to remove than humic/fulvic-like compounds.
At both DWTPs, the average MW decreased two-fold (Tables S5 and S6†) and the change in polydispersity (Δρ) was ∼0.3. Δρ was suggested as both an indicator of coagulation efficiency and a measure of treatability of particular water.2 A large Δρ corresponds to significant narrowing of the MW distribution and thus, indicates efficient removal of compounds in specific MW ranges (for example, large molecules). The values of Δρ calculated for DWTPs A and B were considerably higher than the values reported elsewhere for DWTPs in Norway and Australia.2
The treatment almost completely removed biopolymers (fraction I) and high MW fractions II and III, and reduced fraction IV by ∼90% (see the right columns in Fig. 4 and S6†). Low MW fractions V and VI were removed by ∼80%. Across all the fractions and detection, the lowest removal efficiency (∼20%) was observed for fraction VI monitored by UVA210.
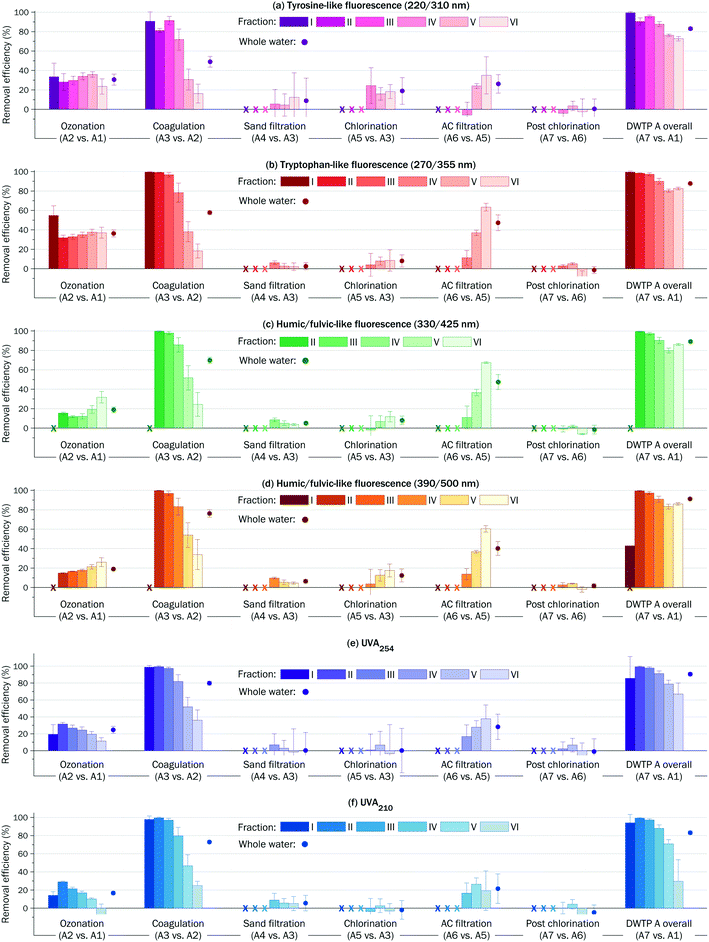 | ||
| Fig. 4 Step-by-step, fraction-by-fraction, and overall efficiency of DWTP A (mean ± sd, n = 4). Completely removed fractions are denoted with ‘x’. | ||
In the samples of treated water, fractions V and VI (MW < 600 Da) contributed to ∼80% of the total tyrosine- and tryptophan-like fluorescence and to ∼70% of the total humic/fulvic-like fluorescence and total UVA (Fig. 3 and S5†). Fraction VI alone contributed to ∼60% of the total UVA210 of the treated waters. This means that low MW compounds represent a major share of DOM in treated water.
For both DWTPs, the chromatographic area of fraction VI detected by UVA210 occasionally was higher for the treated water samples than for the raw water samples, indicating the formation of low MW by-products. Recent studies have demonstrated that DOM of low MW < 1 kDa has DBP formation potential similar to that of high MW humic substances.50–52 And while large hydrophobic humic molecules are readily removed in coagulation, low MW hydrophilic compounds exhibit refractory behavior and pass through conventional water treatment systems. Although DBP identification was out of the scope of this work, the HPSEC-UV-fluorescence approach may be useful in developing surrogate indirect methods to assess DBP formation potential.
3.3. Step-by-step analysis of DWTP performance
The stronger reduction of tyrosine- and tryptophan-like fluorescence (compared to humic/fulvic-like fluorescence), at first glance, contradicts a study where preferential removal of humic-like PARAFAC components was observed during ozonation.19 However, in that study19 ozonation followed coagulation and sand filtration, whereas at DWTP A ozonation is the first pre-treatment step. Hence, the discrepancy may be attributed to differences in DOM composition of the ozonation influents.
Electron withdrawing carboxyl groups formed during pre-ozonation reduce fluorescence quantum yields in aromatic molecules.53 Thus, the observed reduction of fluorescence should not be linearly correlated with possible chemical transformations.
The pre-ozonation did not significantly change the relative abundance of DOM fractions I–VI (compare columns A1 and A2 in Fig. 3). This means that larger molecules were not split into smaller fragments, which would occur at higher ozone doses. However, ozone-induced cleavage of conjugated double bonds (Criegee mechanism) reduced UVA254 of all DOM fractions. The decline of UVA254 was ∼30% for high MW fraction II and ∼15% for low MW fraction VI (Fig. 4e), which reflects the higher aromatic character of humic and fulvic compounds of high MW. The SUVA of whole water after pre-ozonation decreased by ∼20%. A moderate increase in the ratio of UVA210/UVA254 across all the fractions points to formation of aliphatic functional groups (Fig. 5).
The reduction of the total fluorescence and total UVA varied in the range of 60–80%. Fig. 4 and S6† illustrate that high MW fractions I–III were removed almost completely, while intermediate and low MW fractions V and VI were removed by 30–50% and 10–20%, respectively.
Preferential removal of high MW fractions and incomplete removal of low MW fractions in coagulation/flocculation are well-known.24,31,43,44,54–56 In conventional coagulation, insoluble particles are formed as the result of diverse interactions (destabilisation, complexation, entrapment, adsorption) between DOM components and mononuclear, polynuclear, and colloidal species of coagulants. Hydrophobic humic and fulvic compounds, which carry high levels of negative charge due to the presence of ionized carboxyl and phenolic groups, are removed, mainly, through the charge neutralization mechanism. Coagulation of low MW compounds, which are more hydrophilic, occurs, mainly, through adsorption onto colloidal metal hydroxides, which are present at lower concentrations. Thus, hydrophobic high MW fractions are generally more coagulable than hydrophilic low MW fractions.57
The ratio of UVA210/UVA254 can be used as a surrogate indicator of relative hydrophobicity/hydrophilicity to predict the coagulation performance. For both DWTPs, similar correlations were observed between the ratios of UVA210/UVA254 and coagulation efficiencies (Fig. S8†). The most hydrophobic fractions with ratio of UVA210/UVA254 in the range of 1.8–1.9 were readily removed in coagulation by >80%. For fractions with ratio of UVA210/UVA254 ∼ 2.0, the coagulation efficiency was ∼50%. The coagulation efficiency for the most hydrophilic fraction VI (UVA210/UVA254 > 2.8) was <30%.
At DWTP A, the removal efficiencies observed for tyrosine- and tryptophan-like fluorescent compounds in low MW fraction V were ∼10% lower than those at DWTP B. This can be explained in terms of the higher hydrophilicity of the coagulation influent at DWTP A due to the formation of polar carboxyl groups during the pre-ozonation. Alternatively, it is possible that some coagulable compounds in fraction V were removed in the pre-ozonation.
Our results demonstrate that MW determines the coagulation efficiency of both UV absorbing DOM and fluorescent DOM.
At DWTP B, fractions II–III, which were almost completely removed in the preceding coagulation, reappeared after the oxidation with ClO2. The area of fraction IV also increased, however the areas of low MW fractions V and VI did not change or slightly decreased. The apparent formation of high MW DOM is reflected by the negative removal efficiencies in Fig. S6.† This phenomenon was observed at DWTP B a decade ago.23 It is hypothesized that low and intermediate MW compounds could aggregate or undergo polymerization induced by active species formed during the oxidation.23,58 Another possibility is the release of organic compounds during partial oxidation of colloidal matter.43 However, no final explanation has been suggested.
The preferential adsorption of low MW fractions onto AC can be explained on the basis of steric effects: smaller molecules more easily diffuse into pores of AC, while larger molecules have limited access to the pores.59,60
At the same time, fraction VI (MW < 300 Da) exhibited relatively low reduction of UVA210 (∼20% at DWTP A and <10% at DWTP B). This can be attributed to the hydrophilic character of this fraction, which also had the highest ratio of UVA210/UVA254 > 3. In general, hydrophilic molecules do not adsorb well onto AC.23,58,59 Low MW compounds in AC filtration effluents may also represent metabolic products of microbes living in the filters.23,61 However, the microbes would also excrete high MW biopolymers that are poorly retained by AC filters.59,60 And since biopolymer fraction I was not detected in the AC filtration effluents, an intense microbial activity in the AC filters was ruled out.
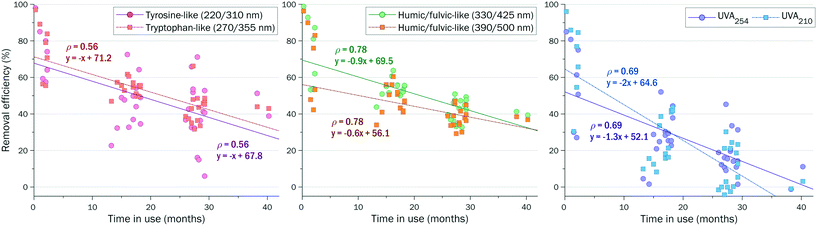
The efficiency of AC filtration declined faster for UV absorbing compounds than for fluorescent compounds (Fig. 6). New or recently regenerated AC retained 50–100% of DOM components. After 30 months, the AC was able to remove <60% of tyrosine- and tryptophan-like compounds, <50% of humic/fulvic-like fluorescent compounds and <30% of UV absorbing compounds.
The efficiency of AC filtration declined simultaneously for fractions IV–VI (Fig. S9†). The fastest decline of AC filtration efficiency (from >80% to <20% in 30 months) was observed for low MW fraction VI detected by UVA210.
This quick assessment demonstrates that HPSEC-UV-fluorescence analysis may be helpful to predict removal efficiencies based on the spectroscopic properties of DOM fractions, to schedule maintenance of AC filters and to monitor possible microbial activity in AC filters (by following tyrosine- and tryptophan-like fluorescence). For long-term monitoring, sampling of the AC filtration effluent should be done immediately after the AC backwash.
4. Conclusions
HPSEC-UV-fluorescence was applied to investigate the transformations of DOM along two conventional DWTPs and to evaluate the removal of protein-like and humic/fulvic-like DOM fractions of various MWs in the main treatment processes: pre-ozonation, coagulation/flocculation, intermediate oxidation, sand filtration, AC filtration, etc.Fluorescent protein-like compounds and humic substances exhibited different removal efficiencies. MW was the main factor determining the efficiency of coagulation/flocculation and AC filtration. While larger molecules were readily removed in the coagulation, the AC filtration favoured removal of smaller molecules. Pre-ozonation of raw water led to a higher decrease in tyrosine- and tryptophan-like fluorescence than in humic/fulvic-like fluorescence. The refractory DOM, which passed through the DWTPs, was present, mainly, in two fractions of MW 500–600 Da and 100–300 Da.
The HPSEC-UV-fluorescence approach allows rapid, robust and sensitive detection, characterization, and tracking of protein-like compounds and humic substances. However, a better understanding of the molecular structures responsible for protein-like and humic/fulvic-like fluorescence is needed to unambiguously assign observed signals to the chemical structures of DOM components. Automatic processing of HPSEC chromatograms, used in this work, allowed fast calculation of various water quality parameters and demonstrated high potential of the HPSEC approach for future on-line monitoring and early warning systems.
Abbreviation
| DOM | Dissolved organic matter |
| HPSEC | High-pressure size-exclusion chromatography |
| DWTP | Drinking water treatment plant |
| MW | Molecular weight |
| DOC | Dissolved organic carbon |
| UVA | UV absorbance |
| AC | Activated carbon |
| EEM | Excitation–emission matrix |
| PARAFAC | Parallel factor analysis |
| λ ex/λem | Excitation/emission wavelength pair |
| M W | Weight average molecular weight |
| M N | Number average molecular weight |
| ρ | Polydispersity |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The first author sincerely appreciates the financial support from Maa- ja vesitekniikan tuki ry (Finland), grant 37141. The authors are thankful to the personnel of DWTPs for the help with water sampling. Constructive comments of the two anonymous reviewers are gratefully acknowledged.References
- K. Katsanou and H. K. Karapanagioti, Surface Water and Groundwater Sources for Drinking Water, in Applications of Advanced Oxidation Processes (AOPs) in Drinking Water Treatment, ed. A. Gil, L. A. Galeano and M. Á. Vicente, Springer International Publishing, Cham, 2017, vol. 67, pp. 1–19, DOI:10.1007/698_2017_140.
- R. Fabris, C. W. K. Chow, M. Drikas and B. Eikebrokk, Comparison of NOM Character in Selected Australian and Norwegian Drinking Waters, Water Res., 2008, 42(15), 4188–4196, DOI:10.1016/j.watres.2008.06.023.
- M. Forsius, A. Räike, I. Huttunen, H. Poutanen, T. Mattsson, S. Kankaanpää, P. Kortelainen and V.-P. Vuorilehto, Observed and Predicted Future Changes of Total Organic Carbon in the Lake Päijänne Catchment (Southern Finland): Implications for Water Treatment of the Helsinki Metropolitan Area, Boreal Environ. Res., 2017, 22, 317–336 Search PubMed.
- M. R. Teixeira and L. M. Nunes, The Impact of Natural Organic Matter Seasonal Variations in Drinking Water Quality, Desalin. Water Treat., 2011, 36(1–3), 344–353, DOI:10.5004/dwt.2011.2524.
- N. Beauchamp, C. Dorea, C. Bouchard and M. Rodriguez, Use of Differential Absorbance to Estimate Concentrations of Chlorinated Disinfection By-Product in Drinking Water: Critical Review and Research Needs, Crit. Rev. Environ. Sci. Technol., 2018, 48(2), 210–241, DOI:10.1080/10643389.2018.1443668.
- S. Richardson, M. Plewa, E. Wagner, R. Schoeny and D. Demarini, Occurrence, Genotoxicity, and Carcinogenicity of Regulated and Emerging Disinfection by-Products in Drinking Water: A Review and Roadmap for Research, Mutat. Res., Rev. Mutat. Res., 2007, 636(1–3), 178–242, DOI:10.1016/j.mrrev.2007.09.001.
- M. J. Lehtola, I. T. Miettinen, T. Vartiainen and P. J. Martikainen, Changes in Content of Microbially Available Phosphorus, Assimilable Organic Carbon and Microbial Growth Potential during Drinking Water Treatment Processes, Water Res., 2002, 36(15), 3681–3690, DOI:10.1016/S0043-1354(02)00100-8.
- R. Srinivasan and G. A. Sorial, Treatment of Taste and Odor Causing Compounds 2-Methyl Isoborneol and Geosmin in Drinking Water: A Critical Review, J. Environ. Sci., 2011, 23(1), 1–13, DOI:10.1016/S1001-0742(10)60367-1.
- G. Abbt-Braun, U. Lankes and F. H. Frimmel, Structural Characterization of Aquatic Humic Substances – The Need for a Multiple Method Approach, Aquat. Sci., 2004, 66(2), 151–170, DOI:10.1007/s00027-004-0711-z.
- A. Matilainen, E. T. Gjessing, T. Lahtinen, L. Hed, A. Bhatnagar and M. Sillanpää, An Overview of the Methods Used in the Characterisation of Natural Organic Matter (NOM) in Relation to Drinking Water Treatment, Chemosphere, 2011, 83(11), 1431–1442, DOI:10.1016/j.chemosphere.2011.01.018.
- A. Ignatev and T. Tuhkanen, Monitoring WWTP Performance Using Size-Exclusion Chromatography with Simultaneous UV and Fluorescence Detection to Track Recalcitrant Wastewater Fractions, Chemosphere, 2019, 214, 587–597, DOI:10.1016/j.chemosphere.2018.09.099.
- H. Huang, E. Sawade, D. Cook, C. W. K. Chow, M. Drikas and B. Jin, High-Performance Size Exclusion Chromatography with a Multi-Wavelength Absorbance Detector Study on Dissolved Organic Matter Characterisation along a Water Distribution System, J. Environ. Sci., 2016, 44, 235–243, DOI:10.1016/j.jes.2015.12.011.
- K. H. Tan, Humic Matter in Soil and the Environment: Principles and Controversies, CRC Press, Taylor & Francis Group, Boca Raton, 2nd edn, 2014 Search PubMed.
- E. M. Thurman, Organic Geochemistry of Natural Waters, Kluwer Academic, 1985 Search PubMed.
- T. Tuhkanen and A. Ignatev, Humic and Fulvic Compounds. Encyclopedia of Analytical Science, Elsevier, 3rd edn, 2019, pp. 411–417, DOI:10.1016/B978-0-12-409547-2.14413-0.
- D. Wang, L. Xing, J. Xie, C. W. K. Chow, Z. Xu, Y. Zhao and M. Drikas, Application of Advanced Characterization Techniques to Assess DOM Treatability of Micro-Polluted and Un-Polluted Drinking Source Waters in China, Chemosphere, 2010, 81(1), 39–45, DOI:10.1016/j.chemosphere.2010.07.013.
- M. Heibati, C. A. Stedmon, K. Stenroth, S. Rauch, J. Toljander, M. Säve-Söderbergh and K. R. Murphy, Assessment of Drinking Water Quality at the Tap Using Fluorescence Spectroscopy, Water Res., 2017, 125, 1–10, DOI:10.1016/j.watres.2017.08.020.
- S. Nowicki, D. J. Lapworth, J. S. T. Ward, P. Thomson and K. Charles, Tryptophan-like Fluorescence as a Measure of Microbial Contamination Risk in Groundwater, Sci. Total Environ., 2019, 646, 782–791, DOI:10.1016/j.scitotenv.2018.07.274.
- S. A. Baghoth, S. K. Sharma and G. L. Amy, Tracking Natural Organic Matter (NOM) in a Drinking Water Treatment Plant Using Fluorescence Excitation–Emission Matrices and PARAFAC, Water Res., 2011, 45(2), 797–809, DOI:10.1016/j.watres.2010.09.005.
- Y. Shutova, A. Baker, J. Bridgeman and R. K. Henderson, Spectroscopic Characterisation of Dissolved Organic Matter Changes in Drinking Water Treatment: From PARAFAC Analysis to Online Monitoring Wavelengths, Water Res., 2014, 54, 159–169, DOI:10.1016/j.watres.2014.01.053.
- B. P. Allpike, A. Heitz, C. A. Joll, R. I. Kagi, G. Abbt-Braun, F. H. Frimmel, T. Brinkmann, N. Her and G. Amy, Size Exclusion Chromatography To Characterize DOC Removal in Drinking Water Treatment, Environ. Sci. Technol., 2005, 39(7), 2334–2342, DOI:10.1021/es0496468.
- Z. Aslam, C. W. K. Chow, F. Murshed, J. A. van Leeuwen, M. Drikas and D. Wang, Variation in Character and Treatability of Organics in River Water: An Assessment by HPAC and Alum Coagulation, Sep. Purif. Technol., 2013, 120, 162–171, DOI:10.1016/j.seppur.2013.09.033.
- A. Matilainen, N. Vieno and T. Tuhkanen, Efficiency of the Activated Carbon Filtration in the Natural Organic Matter Removal, Environ. Int., 2006, 32(3), 324–331, DOI:10.1016/j.envint.2005.06.003.
- A. Matilainen, N. Lindqvist and T. Tuhkanen, Comparison of the Effiency of Aluminium and Ferric Sulphate in the Removal of Natural Organic Matter During Drinking Water Treatment Process, Environ. Technol., 2005, 26(8), 867–876, DOI:10.1080/09593332608618502.
- A. Matilainen, N. Lindqvist, S. Korhonen and T. Tuhkanen, Removal of NOM in the Different Stages of the Water Treatment Process, Environ. Int., 2002, 28(6), 457–465, DOI:10.1016/S0160-4120(02)00071-5.
- T. K. Nissinen, I. T. Miettinen, P. J. Martikainen and T. Vartiainen, Molecular Size Distribution of Natural Organic Matter in Raw and Drinking Waters, Chemosphere, 2001, 45(6–7), 865–873, DOI:10.1016/S0045-6535(01)00103-5.
- H. M. Szabo and T. Tuhkanen, The Application of HPLC–SEC for the Simultaneous Characterization of NOM and Nitrate in Well Waters, Chemosphere, 2010, 80(7), 779–786, DOI:10.1016/j.chemosphere.2010.05.007.
- N. Her, G. Amy, D. Foss and J. Cho, Variations of Molecular Weight Estimation by HP-Size Exclusion Chromatography with UVA versus Online DOC Detection, Environ. Sci. Technol., 2002, 36(15), 3393–3399, DOI:10.1021/es015649y.
- J. K. Edzwald and J. E. Tobiason, Enhanced Coagulation: US Requirements and a Broader View, Water Sci. Technol., 1999, 40(9), 63–70, DOI:10.1016/s0273-1223(99)00641-1.
- T. Vartiainen, A. Liimatainen and P. Kauranen, The Use of TSK Size Exclusion Columns in Determination of the Quality and Quantity of Humus in Raw Waters and Drinking Waters, Sci. Total Environ., 1987, 62, 75–84, DOI:10.1016/0048-9697(87)90484-0.
- S. A. Huber, A. Balz, M. Abert and W. Pronk, Characterisation of Aquatic Humic and Non-Humic Matter with Size-Exclusion Chromatography – Organic Carbon Detection – Organic Nitrogen Detection (LC-OCD-OND), Water Res., 2011, 45(2), 879–885, DOI:10.1016/j.watres.2010.09.023.
- S. H. So, I. H. Choi, H. C. Kim and S. K. Maeng, Seasonally Related Effects on Natural Organic Matter Characteristics from Source to Tap in Korea, Sci. Total Environ., 2017, 592, 584–592, DOI:10.1016/j.scitotenv.2017.03.063.
- G. Becher, G. E. Carlberg, E. T. Gjessing, J. K. Hongslo and S. Monarca, High-Performance Size Exclusion Chromatography of Chlorinated Natural Humic Water and Mutagenicity Studies Using the Microscale Fluctuation Assay, Environ. Sci. Technol., 1985, 19(5), 422–426, DOI:10.1021/es00135a006.
- M. Yan, G. Korshin, D. Wang and Z. Cai, Characterization of Dissolved Organic Matter Using High-Performance Liquid Chromatography (HPLC)–Size Exclusion Chromatography (SEC) with a Multiple Wavelength Absorbance Detector, Chemosphere, 2012, 87(8), 879–885, DOI:10.1016/j.chemosphere.2012.01.029.
- Q. Zhou, Considerations in the Use of High-Pressure Size Exclusion Chromatography (HPSEC) for Determining Molecular Weights of Aquatic Humic Substances, Water Res., 2000, 34(14), 3505–3514, DOI:10.1016/S0043-1354(00)00115-9.
- V. Lepane, L. Depret, A.-L. Väli and K. Suursööt, Impact of Seasonal Climate Change on Optical and Molecular Properties of River Water Dissolved Organic Matter by HPLC-SEC and UV-Vis Spectroscopy, Chem. Biol. Technol. Agric., 2015, 2(14), 1–7, DOI:10.1186/s40538-015-0040-6.
- A. Piccolo, The Supramolecular Structure of Humic Substances, Soil Sci., 2001, 166(11), 810–832, DOI:10.1097/00010694-200111000-00007.
- W.-T. Li, M. Majewsky, G. Abbt-Braun, H. Horn, J. Jin, Q. Li, Q. Zhou and A.-M. Li, Application of Portable Online LED UV Fluorescence Sensor to Predict the Degradation of Dissolved Organic Matter and Trace Organic Contaminants during Ozonation, Water Res., 2016, 101, 262–271, DOI:10.1016/j.watres.2016.05.090.
- J. B. Fellman, E. Hood and R. G. M. Spencer, Fluorescence Spectroscopy Opens New Windows into Dissolved Organic Matter Dynamics in Freshwater Ecosystems: A Review, Limnol. Oceanogr., 2010, 55(6), 2452–2462, DOI:10.4319/lo.2010.55.6.2452.
- N. Senesi, T. M. Miano, M. R. Provenzano and G. Brunetti, Characterization, Differentiation, and Classification of Humic Substances by Fluorescence Spectroscopy, Soil Sci., 1991, 152(4), 259–271 CrossRef CAS.
- Y. Yamashita and E. Tanoue, Chemical Characterization of Protein-like Fluorophores in DOM in Relation to Aromatic Amino Acids, Mar. Chem., 2003, 82(3–4), 255–271, DOI:10.1016/S0304-4203(03)00073-2.
- J. K. Edzwald, Coagulation in Drinking Water Treatment: Particles, Organics and Coagulants, Water Sci. Technol., 1993, 27(11), 21–35, DOI:10.2166/wst.1993.0261.
- J. W. Park, H.-C. Kim, A. S. Meyer, S. Kim and S. K. Maeng, Influences of NOM Composition and Bacteriological Characteristics on Biological Stability in a Full-Scale Drinking Water Treatment Plant, Chemosphere, 2016, 160, 189–198, DOI:10.1016/j.chemosphere.2016.06.079.
- V. L. Quang, I. Choi and J. Hur, Tracking the Behavior of Different Size Fractions of Dissolved Organic Matter in a Full-Scale Advanced Drinking Water Treatment Plant, Environ. Sci. Pollut. Res., 2015, 22(22), 18176–18184, DOI:10.1007/s11356-015-5040-3.
- J. Peuravuori and K. Pihlaja, Molecular Size Distribution and Spectroscopic Properties of Aquatic Humic Substances, Anal. Chim. Acta, 1997, 337(2), 133–149, DOI:10.1016/S0003-2670(96)00412-6.
- S.-N. Nam and G. Amy, Differentiation of Wastewater Effluent Organic Matter (EfOM) from Natural Organic Matter (NOM) Using Multiple Analytical Techniques, Water Sci. Technol., 2008, 57(7), 1009–1015, DOI:10.2166/wst.2008.165.
- N. Her, G. Amy, J. Sohn and U. Gunten, UV Absorbance Ratio Index with Size Exclusion Chromatography (URI-SEC) as an NOM Property Indicator, J. Water Supply: Res. Technol.--AQUA, 2008, 57(1), 35–44, DOI:10.2166/aqua.2008.029.
- N. Her, G. Amy, J. Sohn and U. Gunten, UV Absorbance Ratio Index with Size Exclusion Chromatography (URI-SEC) as an NOM Property Indicator, J. Water Supply: Res. Technol.--AQUA, 2008, 57(1), 35–44, DOI:10.2166/aqua.2008.029.
- H. K. Shon, S. H. Kim, L. Erdei and S. Vigneswaran, Analytical Methods of Size Distribution for Organic Matter in Water and Wastewater, Korean J. Chem. Eng., 2006, 23(4), 581–591, DOI:10.1007/BF02706798.
- I. Kristiana, J. Tan, C. A. Joll, A. Heitz, U. von Gunten and J. W. A. Charrois, Formation of N-Nitrosamines from Chlorination and Chloramination of Molecular Weight Fractions of Natural Organic Matter, Water Res., 2013, 47(2), 535–546, DOI:10.1016/j.watres.2012.10.014.
- J. A. Leenheer and J.-P. Croué, Characterizing Aquatic Dissolved Organic Matter. Understanding the Unknown Structures Is Key to Better Treatment of Drinking Water, Environ. Sci. Technol., 2003, 37(1), 18A–26A CrossRef CAS.
- Y. Zhang, N. Zhang, P. Zhao and Z. Niu, Characteristics of Molecular Weight Distribution of Dissolved Organic Matter in Bromide-Containing Water and Disinfection by-Product Formation Properties during Treatment Processes, J. Environ. Sci., 2018, 65, 179–189, DOI:10.1016/j.jes.2017.03.013.
- Aquatic Organic Matter Fluorescence, ed. P. G. Coble, J. Lead, A. Baker, D. M. Reynolds and R. G. M. Spencer, Cambridge Environmental Chemistry Series, Cambridge University Press, Cambridge, 2014, DOI:10.1017/CBO9781139045452.
- O. Gibert, B. Lefèvre, A. Teuler, X. Bernat and J. Tobella, Distribution of Dissolved Organic Matter Fractions along Several Stages of a Drinking Water Treatment Plant, Journal of Water Process Engineering, 2015, 6, 64–71, DOI:10.1016/j.jwpe.2015.03.006.
- R. Jiao, C. W. K. Chow, H. Xu, X. Yang and D. Wang, Organic Removal Assessment at Full-Scale Treatment Facilities Using Advanced Organic Characterization Tools, Environ. Sci.: Processes Impacts, 2014, 16(10), 2451–2459, 10.1039/C4EM00227J.
- E. L. Sharp, P. Jarvis, S. A. Parsons and B. Jefferson, Impact of Fractional Character on the Coagulation of NOM, Colloids Surf., A, 2006, 286(1–3), 104–111, DOI:10.1016/j.colsurfa.2006.03.009.
- A. Matilainen, M. Vepsäläinen and M. Sillanpää, Natural Organic Matter Removal by Coagulation during Drinking Water Treatment: A Review, Adv. Colloid Interface Sci., 2010, 159(2), 189–197, DOI:10.1016/j.cis.2010.06.007.
- J. Swietlik, U. Raczyk-Stanisławiak, S. Biłozor, W. Ilecki and J. Nawrocki, Adsorption of Natural Organic Matter Oxidized with ClO2 on Granular Activated Carbon, Water Res., 2002, 36(9), 2328–2336, DOI:10.1016/S0043-1354(01)00451-1.
- O. Gibert, B. Lefèvre, M. Fernández, X. Bernat, M. Paraira and M. Pons, Fractionation and Removal of Dissolved Organic Carbon in a Full-Scale Granular Activated Carbon Filter Used for Drinking Water Production, Water Res., 2013, 47(8), 2821–2829, DOI:10.1016/j.watres.2013.02.028.
- S. Velten, D. R. U. Knappe, J. Traber, H.-P. Kaiser, U. von Gunten, M. Boller and S. Meylan, Characterization of Natural Organic Matter Adsorption in Granular Activated Carbon Adsorbers, Water Res., 2011, 45(13), 3951–3959, DOI:10.1016/j.watres.2011.04.047.
- E. Vuorio, R. Vahala, J. Rintala and R. Laukkanen, The Evaluation of Drinking Water Treatment Performed with HPSEC, Environ. Int., 1998, 24(5–6), 617–623, DOI:10.1016/S0160-4120(98)00040-3.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ew00340a |
| This journal is © The Royal Society of Chemistry 2019 |