Channelization of water pathway and encapsulation of DS in the SL of the TFC FO membrane as a novel approach for controlling dilutive internal concentration polarization†
Abstract
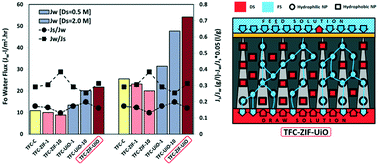
In this study, we explored the use of hydrophobic (ZIF-8) and hydrophilic (UiO-66) water-stable metal–organic-frameworks (MOFs) as well as their mixture for the fabrication of high-performance mixed-matrix membrane (MMM)-based thin film composite (TFC) forward osmosis (FO) membranes for controlling internal concentration polarization (ICP). According to the characteristic curve, the use of UiO-66 inside the support layer (SL) resulted in a negative effect on FO selectivity and positive effect on FO water permeability. Moreover, after the incorporation of ZIF-8 into the SL, the FO selectivity and FO water permeability increased and decreased, respectively. However, the incorporation of the mixture of ZIF-8 and UiO-66 (ZIF-8@UiO-66) into the SL (TFC-ZIF-UiO) caused positive effects on both FO water permeability and selectivity. Channelization and fast water transport through the UiO-66 cavities and the encapsulation of draw solution (DS) into the ZIF-8 cavities are the main reasons for increasing the water flux in the TFC-ZIF-UiO FO membrane. However, the long-time experiment showed that this phenomenon requires time. After replacing DI water with Caspian seawater, the more positive impact of using the TFC-ZIF-UiO membrane is more evident. Therefore, the strategy of using a mixture of hydrophobic and hydrophilic MOFs inside the SL of the TFC FO membranes is of great interest and potential for advancing membrane performance in water and wastewater treatment.
- This article is part of the themed collection: Best Papers 2019 – Environmental Science: Water Research & Technology


 Please wait while we load your content...
Please wait while we load your content...