Membrane distillation crystallization for brine mining and zero liquid discharge: opportunities, challenges, and recent progress
Abstract
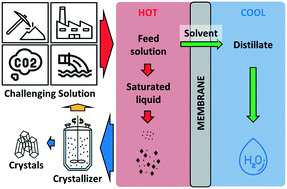
Membrane distillation crystallization (MDC) is an emerging alternative for sustainable management of challenging hypersaline solutions such as seawater brine, produced water, and some industrial wastewaters. MDC combines two individual processes, specifically membrane distillation and crystallization to facilitate the extraction of clean water and separation of high purity salt contents from a brine solution. The potential of MDC for treating challenging hypersaline solutions has been suggested in numerous proof-of-concept studies and some lab-scale demonstration. Nonetheless, there still remain technical challenges which need to be studied before MDC can be economically implemented in practice. This review documents the basic concept of MDC and opportunities to strategically deploy this emerging technology platform for brine mining and zero liquid discharge (ZLD). This paper also discusses key technical challenges (including scaling prevention and membrane wetting which need to be addressed for successful field application) hindering the practical realization of MDC for full-scale operation as well as pathways to address these challenges. Different crystallization techniques such as reaction crystallization and drowning-out crystallization are covered here in order to offer suggestions on their suitability for MDC operation.
- This article is part of the themed collection: Best Papers 2019 – Environmental Science: Water Research & Technology


 Please wait while we load your content...
Please wait while we load your content...