Microplastic biofilm in fresh- and wastewater as a function of microparticle type and size class†
Abstract
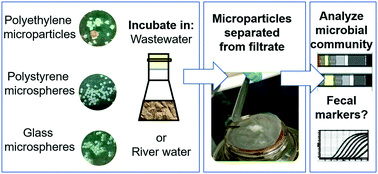
Microplastics are pollutants of concern in the freshwater and marine environments. These microparticles carry biofilm communities unique from the surrounding water. The objective of this study was to investigate the role of source water quality, microparticle type (i.e., different polymers and morphologies), and microparticle size on the resulting biofilm microbial communities. Of particular interest was determining whether microbial agents were concentrated in these biofilm communities. A series of batch reactors were prepared to investigate the microbial communities observed in wastewater or river water compared to those in biofilm on microplastic or glass microspheres via amplicon sequencing. Sampling was performed after 48 h. qPCR was also performed for a fecal indicator organism marker (BacHum) and sul1 antibiotic resistance gene. The biofilm community structures varied as a function of source water and as a function of microparticle type: polystyrene spheres had different microbial community structures from polyethylene microparticles. The differences observed between microparticle types may be due to the morphology/surface texture rather than polymer composition given that some glass microspheres had similar microbial communities to polystyrene microspheres. For a given microparticle type and source water, size (106–125 μm versus 355–425 μm for polystyrene microspheres, 125–250 μm versus 250–500 μm for polyethylene microparticles) did not significantly impact the microbial community structure. While the biofilm microbial communities differed from communities in the surrounding water, the biofilm did not have a higher relative abundance of the indicator organism marker or sul1 genes than the reactor filtrate.
- This article is part of the themed collections: Best Papers of 2019 from RSC’s Environmental Science family journals and Best Papers 2019 – Environmental Science: Water Research & Technology


 Please wait while we load your content...
Please wait while we load your content...