Two-step startup improves pollutant removal in membrane-aerated biofilm reactors treating high-strength nitrogenous wastewater
Rongchang
Wang
 *ab,
Xu
Zeng
ab,
Yanan
Wang
ab,
Tong
Yu
c and
Zbigniew
Lewandowski
d
*ab,
Xu
Zeng
ab,
Yanan
Wang
ab,
Tong
Yu
c and
Zbigniew
Lewandowski
d
aKey Laboratory of Yangtze Aquatic Environment (MOE), College of Environmental Science and Engineering, Tongji University, Shanghai, 200092, China. E-mail: wangrongchang@tongji.edu.cn
bInstitute of Biofilm Technology (IBT), State Key Laboratory of Pollution Control and Resource Reuse, Shanghai Institute of Pollution Control and Ecological Security, Tongji University, Shanghai, 200092, China
cDepartment of Civil and Environmental Engineering, Alberta University, Edmonton, Alberta T6G 2M8, Canada
dCenter for Biofilm Engineering, Montana State University, Bozeman, MT 59717, USA
First published on 22nd October 2018
Abstract
The goal of this study is to develop a two-step startup strategy for establishing a layered biofilm in membrane-aerated biofilm reactors (MABRs) treating strong nitrogenous wastewater. We hypothesized that using a two-step startup strategy based on the deliberate deposition of nitrifiers on the membrane in the first step and of heterotrophs away from the membrane in the second step would improve the removal rates of ammonia and COD compared to a one-step startup procedure. The results demonstrate that MABRs with a two-step startup had more than double the specific removal rates of ammonia and COD compared with the reactors using a one-step startup. Direct use of biofilm inoculum exposed to high ammonium concentration can shorten the startup duration. It was found that the abundance of the amoA gene in biofilms from MABRs with nitrifying biofilm inoculum was two orders of magnitude higher than that with nitrifying sludge inoculum after 63 days of operation. The sequences for introducing COD in the second step of the startup either stepwise or gradually had less pronounced effects on the rates of substrate removal. The provided startup strategy is beneficial for practical operation of MABRs for treating high-strength nitrogenous wastewater.
Water impactMembrane-aerated biofilm reactors (MABRs) are favorable for simultaneous nitrification and denitrification due to microbial population stratification along the oxygen gradient in the biofilm. A rational and efficient two-step startup strategy is suitable for MABRs treating strong nitrogenous wastewaters. MABRs with a two-step startup facilitate the formation of layered distribution of nitrifiers and denitrifiers in biofilm and have higher ammonia and COD removal rates and a shorter startup duration time. |
1. Introduction
Ammonium pollution, which can cause eutrophication and be toxic to aquatic species, is becoming a serious environmental problem.1 The conventional approach to treating high-strength ammonia wastewater relies on aerobic nitrification and anoxic denitrification:2,3 aerobic nitrification with the terminal conversion of NH4+-N to NO3−-N and the subsequent anoxic denitrification with the conversion of NO3−-N to molecular nitrogen.4 It is well known that it is difficult to combine these two processes in a single reactor because they employ groups of organisms with dramatically different requirements for growth: slowly growing aerobic autotrophic nitrifiers and rapidly growing anoxic heterotrophic denitrifiers. This difficulty is, to some extent, alleviated in biofilm reactors, because the attached growth of microorganisms allows a practically unlimited solid retention time (SRT), which removes the dependence on the hydraulic retention time (HRT). Biofilm reactor technology has provided us with a useful configuration known as the membrane-aerated biofilm reactor (MABR), in which biofilms are aerated through a gas-permeable membrane, which economizes the most expensive process in traditional wastewater treatment, aeration.5–7 The interest in MABRs stems from their low energy requirements.8In the MABR, a biofilm is naturally immobilized on an oxygen-permeable membrane. Mechanistically, oxygen is delivered through the membrane to the bottom of the biofilm, where oxygen is utilized by bacteria to oxidize COD and/or ammonia, which are delivered through the biofilm/bulk water interface. Such an arrangement in which the oxidant and the reducer are delivered from opposite sides of the biofilm is commonly known as counter-diffusion.9,10 The oxygen supply rate can be controlled through the intramembrane oxygen partial pressure and the membrane surface area.11,12 Pure oxygen instead of air can be supplied through the membrane and the contact time between oxygen and the biofilm can be increased to obtain close to 100% oxygen conversion. It was reported that membrane oxygen transfer efficiencies (OTEs) as high as 100% were achieved in a composite membrane bubbleless aerator.13 Casey reported that membrane-aerated biofilm reactors, which operated with higher thicknesses of active biomass than conventional biofilm reactors, offered the advantage of close to 100% oxygen conversion efficiencies for the treatment of high-strength wastewaters.14 A pilot-scale MABR was successfully employed to treat landfill leachate with influent ammonium concentrations ranging from 500 to 2500 mg L−1; 80–99% nitrification was achieved.15
MABR has the advantage of higher COD removal rates than conventional biofilm technologies, such as rotating biological contactors (RBCs) and biological aerated filters (BAFs).16 Pankhania reported that the organic carbon removal fluxes could be up to 42.7 g COD per m2 per d in an MABR with polypropylene sealed-end membranes.17 Brindle investigated pilot-scale MABRs operated for 90 days treating high-strength wastewater from cider manufacturing.6 The dissolved COD removal efficiencies were over 90%, with steady-state removal fluxes at the highest loading rates of 62.6 g COD per m2 per d (at HRT 1.4 h), and 60.4 g COD per m2 per d (at HRT 1.8 h). It was also found that the MABR could achieve 95% COD removal at 10 g COD per m2 per d.18
Because of the unique microbial stratification of biofilm in the MABR, nitrification, denitrification, and COD removal could exist in a single biofilm.15,19–21 Nitrifiers are preferentially located in the oxygen-rich region adjacent to the membrane/biofilm interface, whereas denitrifiers grow in the anoxic region at the biofilm/liquid interface, where the COD concentration is typically at its highest value. Terada et al. reported a total nitrogen removal rate of 96% in a sequencing batch membrane biofilm reactor.22 Downing and Nerenberg reported a total nitrogen removal of 75% and a nitrification rate of 0.85 g N per m2 per d in a hybrid membrane biofilm reactor.23 Downing reported a stable nitritation in a continuously aerated MABR with a short HRT of less than 60 days.24 MABRs can favor the growth of anammox bacteria through a variety of oxygen control strategies designed to repress nitrite-oxidizing bacteria activity, including control of the oxygen/nitrogen surface loading ratio25–27 and control of the DO concentration gradient at the membrane/biofilm interface.28
The layered anoxic and aerobic microenvironments in membrane-aerated biofilms are beneficial for simultaneous nitrification and denitrification because the thickness of the anoxic and aerobic layers can be controlled via transmembrane gas pressure. LaPara's results demonstrated that ammonia-oxidizing bacteria grow near the membrane, while denitrifying bacteria grow at a substantial distance from the membrane. Nitrifying and denitrifying bacteria did not grow simultaneously when organic concentrations became too high or ammonia concentrations became too low.29 It is necessary both to understand and ultimately to control the microenvironment in the biofilm: this will allow optimization of the community structure and spatial organization in the biofilm and consequently high nitrogen removal efficiency.21 The ability of the MABR to retain the slow-growing nitrifying bacteria in the biofilm along with its ability to supply oxygen directly to them makes it ideally suited for these applications.30 However, researchers also report difficulties in establishing sustained operational conditions.15,20 Even though these authors explicitly refer to the importance of the different layers in the biofilm and their positions, little has been done to actually control the locations of the layers.11 It is easy to deduce that implementing effective control over the position and thickness of the active layers of the microorganisms, autotrophs and heterotrophs deposited on the surface of the membrane would improve the performance of the reactor.
The traditional approach to starting up an MABR for nitrification and denitrification is to encourage the growth of both groups of microorganisms, autotrophic nitrifiers and heterotrophic denitrifiers, on the surface of the aerating membrane in one step, simultaneously inoculating the membrane with the autotrophs and the heterotrophs. Once the biofilm is established, successful operation of the reactor depends on the precisely controlled delivery of oxygen through the membrane so that the biofilm layers near the membrane are aerated and the layers away from the membrane are not. Although convenient and successful, this procedure does not develop the optimal structure of the biofilm, since the faster growing heterotrophs are deposited in proximity to the membrane together with the autotrophs and therefore the two groups of organisms compete for oxygen, potentially lowering the nitrification potential. It would be better, at least hypothetically, to put the reactor into operation by first encouraging the growth of nitrifiers in proximity to the membrane and then grow the layer of heterotrophs on top of the nitrifying biofilm, at a distance from the membrane, in a two-step startup procedure. Ideally, the nitrifiers should oxidize ammonia while using most or all of the oxygen delivered through the membrane and the heterotrophs should oxidize the COD delivered from the bulk solution while reducing the nitrates generated by the nitrifiers. It is well known that such a layered structure of the biofilm is needed to treat high-strength ammonia wastewater. However, little effort has been devoted to developing startup procedures that control the locations of these active layers.
The goal of the present study is to implement a two-step startup strategy for establishing stratified biofilm on the membranes in MABRs treating strong nitrogenous wastewater and compare the substrate removal rates and startup durations of one-step and two-step startup strategies for MABRs.
2. Materials and methods
2.1. Reactors
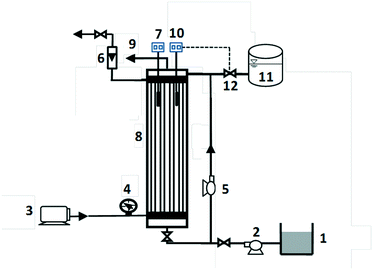
A diagram of a bench-scale membrane-aerated biofilm reactor (MABR) we used in this study is shown in Fig. 1. The reactor was made of a polycarbonate tube 255 mm long and 26 mm in diameter. The wastewater was delivered using a peristaltic pump (Ismatech, MV-MS/CA8C, Glattburg, Zürich, Switzerland) to the bottom of the reactor, and the effluent was removed from the top. The effective volume of the system, including the tubing, was 0.18 L, and the hydraulic retention time (HRT) was 2.8 d. Oxygen was delivered using a gas-permeable polydimethylsiloxane (PDMS) membrane (Shanghai Yiyan Rubber Products Co. Ltd.). The inner diameter of the silicone membrane was 2.0 mm, and the outer diameter was 3.0 mm. The membrane module consisted of 4 nonporous silicone membranes with a total surface area of 0.0045 m2 and a specific surface area of 25.13 m2 m−3. The mass transfer coefficient for oxygen delivery, kLa, was 0.81 m d−1, and the oxygen pressure in the membrane lumen was 20 kPa. The reactor was backmixed via recirculation with an average flow velocity of 1.25 cm s−1. Five MABRs were operated in parallel.2.2. Medium
The synthetic wastewater was composed of ammonium bicarbonate, NH4HCO3; potassium phosphate, KH2PO4; sodium acetate, CH3COONa; calcium chloride, CaCl2·2H2O; and magnesium, MgSO4·7H2O. The concentrations of these components varied among the experiments and are reported for the individual reactors. In addition, the feed contained 0.02 g of phosphorus (P) per L and 1 mL L−1 of a trace element solution.312.3. Chemical analysis
Suspended solids (SS), COD, NH4+-N, NO2−-N, and NO3−-N were measured according to standard methods (APHA, 2012). Dissolved oxygen (DO) in bulk liquid was measured with a DO electrode (YSI Model 5331, Yellow Springs, OH, USA). The pH in the bulk liquid in each MABR was kept at 7.5–7.8 by adding 0.2 M NaHCO3 solution using a pH controller (SIN-PH160, Sinomeasure, Hangzhou, Zhejiang, China).The pollutant removal efficiency was calculated using the equation below:
| Removal efficiency (%) = (1 − Ce/C0) × 100 | (1) |
Specific surface removal rates (SRR) of MABR for ammonium and COD are formulated as
| SR = Q(C0 − Ce)/A | (2) |
2.4. Startup strategies
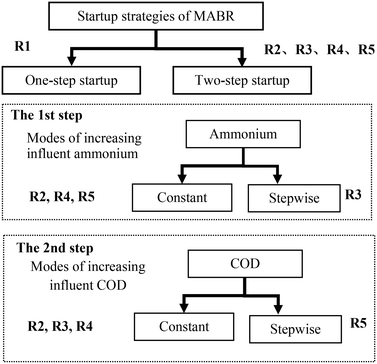
We operated five reactors to quantify the effects of reactor inoculation and the mode of ammonia and COD delivery with their concentrations either fixed or gradually increasing. We hypothesized that a two-step startup strategy, in which two layers of microorganisms are deposited sequentially, would provide better reactor operation than a one-step startup, in which both groups are deposited simultaneously. The strategy for testing this hypothesis is shown in Fig. 2.As shown in Fig. 2, reactor #1 (R1) was operated using a one-step strategy. Reactors #2 to #5 were operated using various two-step startup strategies. For reactor #2 (R2), influent with constant NH4+-N only was provided in the first step and constant NH4+-N and COD were provided in the second step. For reactor #3 (R3), influent with NH4+-N only, increased stepwise, was provided in the first step and constant COD and NH4+-N were provided in the second step. For reactor #4 (R4), influent with constant NH4+-N only was provided in the first step and constant COD and NH4+-N were provided in the second step. For reactor #5 (R5), influent with constant NH4+-N only was provided in the first step and constant NH4+-N and gradually increased COD were provided in the second step.
To investigate the effect of the inoculum on MABR startup, two inocula (Inoculum A and Inoculum B) were used for the five MABRs. R1, R2 and R3 were inoculated with Inoculum A, which was activated sludge from a lab-scale nitrifying membrane bioreactor (MBR) fed with synthetic wastewater (NH4+-N 250 mg N per L and no COD). Inoculum B was biomass from a lab-scale nitrifying membrane-aerated biofilm reactor (MABR) fed with synthetic wastewater (NH4+-N 900 mg N per L and no COD) and was used for R4 and R5. The startup duration was defined as the time needed for the substrate removal efficiency to achieve stability over three consecutive measurements.
2.5. Startup strategies
To collect biofilm samples, the pumps were stopped periodically. One strand of the hollow-fibre silicone membrane was removed and replaced, and a 2.0 cm terminal end was cut off. The sample was immediately fixed in a 4% paraformaldehyde solution (pH 7.2) for 3 h. After fixation, the biofilm samples were submerged overnight in Tissue-Tek® O.C.T.™ Compound (Sakura Finetek Europe B.V., the Netherlands). The sample was frozen at −25 °C and sectioned into 10 mm-thick vertical slices with a cryomicrotome (Reichert-Jung Cryocut 1800, Leica). The produced slices were mounted on microscope slides. Washing and dehydration were performed according to reported procedures.32 The mounted biofilm samples were stained with Syto9 (Molecular Probes Inc., USA) and then observed under a confocal scanning laser microscope (CLSM) (Leica TSC SP5, Germany). The biofilm thickness was measured directly with the CLSM, and the average biofilm thickness was calculated with at least 50 positions for each sample. A shrinking factor (a) of 1.89 was calculated by comparing the biofilm thicknesses before and after the fixation procedure. The shrinking factor was then used to estimate actual biofilm thicknesses from biofilm thicknesses based on staining and CLSM observation.2.6. Microbial activity
Microbial activity was quantified by measuring the specific ammonium oxidation rate (SAOR) and specific denitrification rate (SDNR) in a batch reactor. The results are reported per unit of biofilm biomass as g NH4+-N per g SS per d and g NO3−-N per g SS per d.A 2.0 cm-long piece of hollow-fiber membrane was sampled, as described in section 2.3, and placed in a conical beaker (100 mL), and the biomass was dispersed using a vortex oscillator. The sample was diluted to 50 mL and the beaker was placed on a rotary shaker operated at 180 rpm at 28 ± 1 °C. To measure the SAOR, we used the initial substrate concentrations of 191 mg L−1 NH4Cl and 168 mg L−1 Na2CO3 in the aerated sample. To measure SDNR, we used the initial substrate concentrations of 300 mg L−1 NaNO3 and 400 mg L−1 COD without aeration but with stirring. The tests were run for 3 hours; every 30 min, 2.0 mL of solution was sampled and filtered through a 0.45 μm membrane. The filtered samples were diluted to 10 mL, and the final concentrations of NH4+-N and NO3−-N were measured.33
2.7. Quantification of functional genes with real-time qPCR
Real-time quantitative PCR (qPCR) was performed to quantify the abundance of functional genes, such as amoA, Nitrospira 16S rDNA, narG, nirS and nirK. The primers used for real-time qPCR are presented in Table 2. Ammonia-oxidizing bacteria (AOB) were quantified by targeting a sequence of their amoA gene coding for a subunit of the ammonia monooxygenase, using the primers amoA-1F and amoA-2R.34 Nitrite-oxidizing bacteria (NOB) were quantified by targeting Nitrospira 16S rDNA with the primers NSR1113f and NSR1264r.35 The reduction of nitrate to nitrite can be catalyzed by the membrane-bound nitrate reductase, which is coded by the narG gene.36 The primers narG 1960m2f and narG 2050m2r were used to amplify a 110 bp narG fragment from the targeted cluster.37 The reduction of nitrite to nitric oxide, which distinguishes denitrifiers from other nitrate-respiring bacteria, is catalysed by two different types of nitrite reductases (Nir), either a copper-containing enzyme encoded by nirK or a cytochrome cd1 enzyme encoded by nirS. The fragments of the denitrification gene nirK were amplified with the primer pair nirK 583f and nirK 909r, while the fragments of the nirS gene were amplified with the primer pair nirS 832f and nirS 1606r.38The difference in the abundance of main functional genes related to nitrification and denitrification determined using real-time qPCR in MABRs with different nitrifying bacteria inoculations reveals the difference in microbial composition between these systems. Total DNA was extracted from all samples using the FastDNA® Spin Kit for Soil (MP Biomedicals, Solon, OH) according to the manufacturer's instructions. Extracted DNA from all samples was quantitatively analyzed using the Qubit® dsDNA HS Assay Kit (Invitrogen, USA). Subsequently, samples were diluted to the same concentration, 1.0 ng μl−1. For the quantification of different bacterial groups, amplification was performed in 25 μL reaction mixtures in MicroAmp Optical 96-well reaction plates with optical caps39 using buffers supplied with the qPCR Core Kit for Syber Green I (iQ™ SYBR® Green Supermix; Bio-Rad, Hercules, CA) as described by the suppliers. Triplicate samples were run on a thermocycler ABI 7500 (Applied Biosystems, Singapore). The conditions used for qPCR are as follows: 30 s at 95 °C, 40 cycles at 95 °C for 5 s and 57 °C for 10 s. The qPCR specificity was verified from the melting curve, and the qPCR efficiency was higher than 90% for all samples.
2.8. Statistical analysis
All measurements were conducted in triplicate, and the results are expressed as mean ± standard deviation. An analysis of variance (ANOVA) was performed via SPSS Statistics 17.0 and the Origin 8.0 software (OriginLab Corporation, USA) to test the significance of the results, and p < 0.05 was considered to be statistically significant.3. Results
3.1. One-step versus two-step startup
To compare one-step and two-step startup strategies, we operated two MABRs, R1 and R2. The results for COD and ammonium nitrogen removal efficiency are presented in Fig. 3. A COD removal efficiency of 55.8 ± 4.9% and an NH4+-N removal efficiency of 17.8 ± 3.0% were reached in R1. Much higher substrate removal efficiencies were reached in R2: a COD removal efficiency of 77.3 ± 7.5% and an NH4+-N removal efficiency of 38.3 ± 3.7%.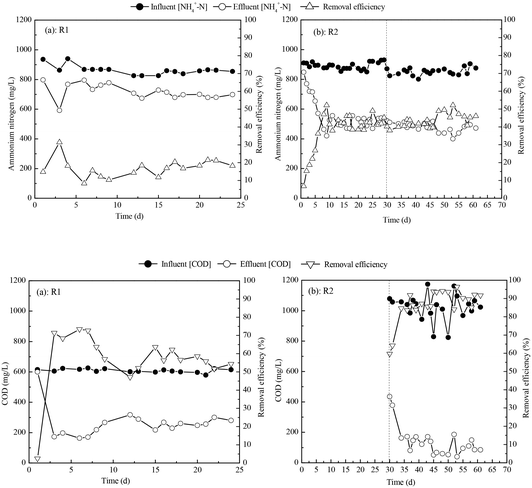 | ||
| Fig. 3 Ammonium and COD removal in MABRs: one-step vs. two-step startup. (a) Ammonium removal in R1; (b) ammonium removal in R2; (c) COD removal in R1; (d) COD removal in R2. | ||
SRRs for ammonia and COD in MABRs operated using various startup strategies are given in Table 1. The SRR for COD was 4.78 ± 0.41 g COD per m2 per d and the SRR for ammonia nitrogen was 2.15 ± 0.39 g N per m2 per d in R1. In R2, during the first step, the SRR for ammonia nitrogen was 5.30 ± 1.54 g N per m2 per d; during the second step, the SRR for ammonia nitrogen was 4.62±0.48 g N per m2 per d and the SRR for COD was 11.30 ± 1.33 g COD per m2 per d. These results show unequivocally that using the two-step startup procedure dramatically improves the performance of the reactors by more than doubling the ammonia and COD removal rates, which supports our hypothesis.
| Reactor | Inoculum | One-step startup | Two-step startup | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Influent: NH4+-N and COD | 1st step influent: NH4+-N only | 2nd step influent: NH4+-N and COD | |||||||||
| NH4+-N SRR g N per m2 per d | TN SRR g N per m2 per d | COD SRR g COD per m2 per d | Duration of the first step (d) | NH4+-N SRR g N per m2 per d | TN SRR g N per m2 per d | Duration of the second step (d) | NH4+-N SRR g N per m2 per d | TN SRR g N per m2 per d | COD SRR g COD per m2 per d | ||
| Inoculum: A: Microorganisms from a lab-scale nitrifying membrane bioreactor (MBR) fed with synthetic wastewater; NH4+-N 250 mg N per L and no COD. B: Microorganisms from a lab-scale nitrifying membrane-aerated biofilm reactor (MABR) fed by synthetic wastewater; NH4+-N 900 mg N per L and no COD. SRR: specific removal rate, g N per m2 per d or g COD per m2 per d, respectively. n.a. – not applicable. | |||||||||||
| R1 | A | 2.15 ± 0.39 | 1.25 ± 0.34 | 4.78 ± 0.41 | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| R2 | A | n.a. | n.a. | n.a. | 23 | 5.30 ± 1.54 | 0.34 ± 0.60 | 9 | 4.62 ± 0.48 | 0.13 ± 0.53 | 11.30 ± 1.33 |
| R3 | A | n.a. | n.a. | n.a. | 33 | 5.31 ± 0.26 | 1.21 ± 0.87 | n.a. | n.a. | n.a. | |
| R4 | B | n.a. | n.a. | n.a. | 8 | 5.38 ± 0.47 | 0.98 ± 0.59 | 7 | 5.28 ± 0.52 | 1.21 ± 0.34 | 12.50 ± 0.49 |
| R5 | B | n.a. | n.a. | n.a. | 10 | 5.49 ± 0.28 | 0.93 ± 0.30 | 30 | 5.34 ± 0.23 | 1.68 ± 1.21 | 12.85 ± 0.62 |
3.2. Effect of the modes of increasing the influent ammonium concentration
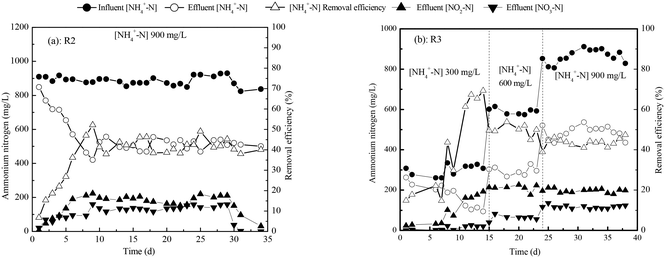
To compare the effects of the modes of increasing the influent ammonium concentration in the first step of a two-step startup, we compared the ammonia removal efficiencies and rates in R2 and R3. Fig. 4 illustrates the ammonia removal efficiencies in reactors R2 and R3. In R2, influent NH4+-N was about 900 mg L−1. In R3, influent NH4+-N increased in three stages, from 0 to 300 mg L−1, to 600 mg L−1, and to 900 mg L−1. As shown in Fig. 4, the startup duration was 23 days for R2 and 33 days for R3. After startup, the NH4+-N removal efficiency was 45.2 ± 4.0% in R2 and 44.0 ± 1.8% in R3. The SRR for ammonia nitrogen was 5.30 ± 1.54 g N per m2 per d in R2 and 5.31 ± 0.26 g N per m2 per d in R3. These results show that there is no significant difference between reactors R2 and R3 in terms of ammonia removal efficiency or SRR. However, the startup time for R2 was shorter.3.3. Effects of the mode of increasing the influent COD concentration
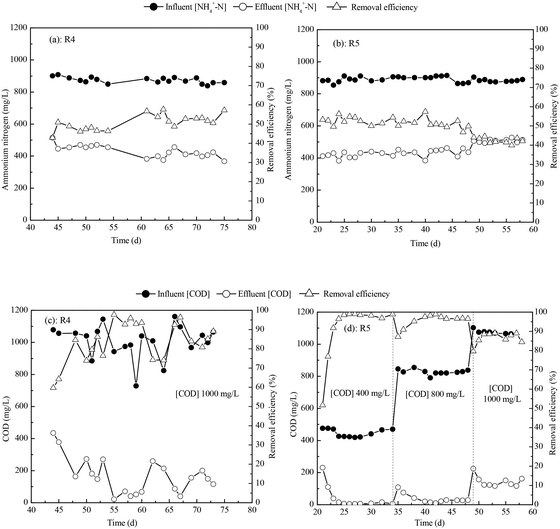
To quantify the effects of the modes of increasing the influent COD concentration during the second step, we compared the ammonia and COD removal efficiencies and rates for R4 and R5. Fig. 5 illustrates the ammonia and COD removal efficiencies in reactors R4 and R5. In R4, influent NH4+-N was kept constant at about 900 mg L−1 and influent COD was kept constant at about 1000 mg L−1. In R5, influent NH4+-N was kept constant at about 900 mg L−1 and influent COD was increased in three stages, from 0 to 400 mg L−1, to 800 mg L−1, and to 1000 mg L−1. The duration of the second step was 7 days for R4 and 30 days for R5. An NH4+-N removal efficiency of 43.2 ± 5.2% was reached in R4 and one of 42.5 ± 1.5% was reached in R5, as shown in Fig. 5(a) and (b). The COD removal efficiency reached 82.1 ± 6.8% in R4 and 86.5 ± 3.1% in R5 (see Fig. 5(c) and (d)). The SRR for ammonia nitrogen was 5.28 ± 0.52 g N per m2 per d in R4 and 5.34 ± 0.23 g N per m2 per d in R5. The SRR for COD was 12.50 ± 0.49 g COD per m2 per d in R4 and 12.86 ± 0.62 g COD per m2 per d in R5. These results show that there is no significant difference between reactors R4 and R5 in terms of ammonia or COD removal efficiency or SRR for ammonia or COD. However, the startup time for R4 was shorter. Our results with reactors R4 and R5 demonstrate that their COD removal efficiency was above 80% and the SRR for COD was above 12.00 g COD per m2 per d.3.4. Effect of choice of inoculum on MABR startup
To quantify the effect of the choice of inoculum on the MABR startup, we compared the ammonia and COD removal efficiencies and rates in R2 and R4. The main difference between these two reactors was the source of inoculated biomass. The duration of the first step was 23 days in R2 and 8 days in R4. The duration of the second step was 9 days in R2 and 7 days in R4. The total startup duration was 32 days for R2 and 15 days for R4.The SRR for ammonia was 4.62 ± 0.48 g N per m2 per d in R2 and 5.28 ± 0.52 g N per m2 per d in R4. SRR for COD was 11.30 ± 1.33 g COD per m2 per d in R2 and 12.85 ± 0.62 g COD per m2 per d in R4, as shown in Table 1. There was no significant difference between reactors R2 and R4 in terms of SRR for ammonia or COD. However, R4 had a shorter startup duration. As shown in Table 3, the specific ammonium oxidation rate (SAOR) was 0.27 ± 0.01 g NH4+-N per g SS per d for the biomass from R2 and 0.36 ± 0.01 g NH4+-N per g SS per d for the biomass from R4, which indicates that the biomass from R4 had a higher ammonium oxidation potential. Therefore, it can be concluded that direct use of the acclimated biofilm inoculum from the laboratory-scale nitrifying MABR (Inoculum B), which was exposed to a high concentration of ammonium, resulted in an increased initial ratio of AOB that had adapted to a high ammonium concentration and that this shortened the startup duration for a MABR treating strong ammonium and COD wastewater, in comparison to inoculation with typical nitrifying activated sludge (inoculum A) which was exposed to a low concentration of ammonium.
In addition, we measured the specific denitrification rate (SDNR) for biomass from R2 and R4, as shown in Table 2. It was 0.39 ± 0.01 g NO3−-N per g SS per d for the biomass from R2 and 0.47 ± 0.02 g NO3−-N per g SS per d for the biomass from R4. It was also found that achieving stable denitrification can be difficult because of the challenge of precisely controlling oxygen flux to the biofilm, even if the abundance of denitrifiers in the biofilm is not a limiting factor.
| Target gene | Primer | Primer sequence | Ref. |
|---|---|---|---|
| amoA | amoA-1F | GGGGTTTCTACTGGTGGT | 34 |
| amoA-2R | CCCCTCKGSAAAGCCTTCTTC | ||
| Nitrospira 16S rDNA | NSR 1113f | CCTGCTTTCAGTTGCTACCG | 35 |
| NSR 1264r | GTTTGCAGCGCTTTGTACCG | ||
| narG | narG 1960m2f | TAGTGGGCAGGAAAACTG | 37 |
| narG 2050m2r | CGTAGAAGAAGCTGGTGCTGTT | ||
| nirK | nirK 583f | TCATGGTGCTGCCGCGGACGG | 38 |
| nirK 909r | GAACTTGCCGGTGCCCAGAC | ||
| nirS | nirS 832f | TCACACCCCGAGCCGCGCGT | 38 |
| nirS 1606r | AGKCGTTGAACTTKCCGGTCGG |
3.5. Abundance of functional genes
The abundances of different functional genes (amoA, Nitrospira 16S rDNA, narG, nirS and nirK) in biofilms from MABRs with different startup strategies were compared and presented in Fig. 6. Biofilm was sampled on day 63 from R2 and R4. Both R2 and R4 were operated with a two-step startup: the first step with influent containing only NH4+-N (without COD) and the second step with influent containing both NH4+-N and COD. R2 was inoculated with typical nitrifying activated sludge exposed to a low concentration of ammonium (Inoculum A), and R4 was inoculated with the acclimated biofilm from an MABR fed with strong ammonium wastewater (Inoculum B).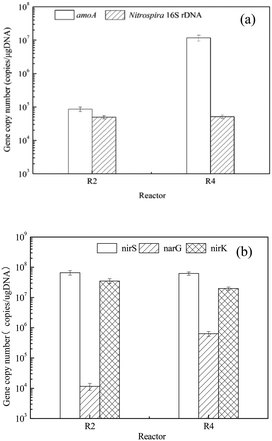 | ||
| Fig. 6 Abundance of functional genes in biofilms from MABRs with different startup strategies: (a) amoA and Nitrospira 16S rDNA; (b) narG, nirS and nirK. | ||
As shown in Fig. 6(a), the abundances of the amoA gene in R2 and R4 were 8.61 × 104 and 1.17 × 107 copies per μg DNA, respectively. It can be seen that the abundance of the amoA gene in R4 was higher than that in R2 by at least two orders of magnitude. Comparing with the functional gene of ammonia-oxidizing bacteria (AOB), the contents of the functional gene of nitrite-oxidizing bacteria (NOB) represented by Nitrospira 16S rDNA in the reactors R2 and R4 were closer. The amounts were 5.14 × 104 and 4.93 × 104 copies per μg DNA, respectively. These results were consistent with the ammonia removal performance in both reactors, such as the SRR for ammonia, 4.62 g N per m2 per d in R2 and 5.28 g N per m2 per d in R4, and the results of the SAOR, 0.27 g NH4+-N per g SS per d for the biomass from R2 and 0.36 g NH4+-N per g SS per d for the biomass from R4, as shown in Table 2. The higher ammonium oxidation potential in R4 can be attributed to the higher abundance of amoA genes in the biofilms. A similar positive correlation between the specific ammonia oxidation rate and the abundance of amoA gene copies was reported in an MABR system treating synthetic domestic wastewater40 and in a redox stratified membrane biofilm reactor (RSMBR) treating ammonium-rich wastewater.41
Fig. 6(b) illustrates the abundance distribution of denitrification functional genes in R2 and R4. The reduction of nitrate to nitrite can be catalyzed by the membrane-bound nitrate reductase, which is coded by the narG gene. The abundance of the narG gene in R2 and R4 was 1.15 × 104 and 6.36 × 106 copies per μg DNA, respectively. We can see that the abundance of the narG gene in R2 was lower than that in R4 by two orders of magnitude. The contents of the denitrification functional gene nirS in R2 and R4 were 6.68 × 107 and 6.22 × 107 copies per μg DNA separately. Similar levels of abundance of the denitrification functional gene nirK were detected, which were 3.53 × 107 and 1.99 × 107 copies per μg DNA, respectively. The higher content of the narG gene is consistent with the higher denitrification activity, expressed by the specific denitrification rate (SDNR), in R4, which was inoculated with the biomass acclimated to strong ammonium wastewater.
4. Discussion
Although extensive studies have investigated MABRs for treating strongly nitrogenous wastewater, to our knowledge, two-step startup strategies have not been attempted. In our study, we hypothesized that using a two-step startup strategy based on the deliberate deposition of nitrifiers near the aerating membrane in the first step and of heterotrophs away from the membrane in the second step would improve ammonia and COD removal rates, compared to reactors put into operation using a one-step start-up procedure, with which it is difficult to develop such a stratified biofilm.The advantages of a two-step startup in MABR are:
(1) Improved substrate removal rates. The results indicate that the startup procedure for MABRs used for COD and nitrogen removal can be improved. Compared with the conventional one-step startup method for MABRs, the two-step startup strategies can reach higher ammonia and COD removal rates. Terada reported that a specific nitrogen removal rate per membrane surface area of about 4.48 g N per m2 per d was obtained for the treatment of high-strength nitrogenous wastewater (Terada et al., 200320). In contrast, our results showed that the SRR for ammonia nitrogen was 5.30 ± 1.54 g m−2 d−1 in R2 and 5.31 ± 0.26 g N per m2 per d in R3. Our results with reactors R4 and R5 demonstrate a COD removal efficiency of above 80% and an SRR for COD of above 12.0 g COD per m2 per d.
(2) Shortened startup duration. For the two-step startup strategies, there is no need to increase the influent NH4+-N or COD loading gradually. Our results show that nitrifying bacteria can grow by apposition after seven days and the ammonia loading can be increased up to 5.4 ± 0.5 g N per m2 per d. In contrast, Downing reported a stable nitritation in continuously aerated MABRs with durations of less than 60 days.42 On the other hand, there is no need to decrease the COD loading. The growth and development of heterotrophic denitrifying bacteria could be enhanced by the higher COD loading. Thus, the MABR could be used for the treatment of raw high-strength nitrogenous wastewater directly. Therefore, the two-step startup strategies shorten the startup duration significantly.
(3) Enhanced layering of biofilm structure. In contrast with the conventional one-step startup method for MABRs, the two-step startup strategies favor the formation of a layered biofilm structure, with the heterotrophic denitrifying bacteria at the surface of the biofilm and the nitrifying bacteria at the bottom of the biofilm (Fig. 7). A layered biofilm can provide aerobic and anoxic conditions simultaneously within a single biofilm,18,43 allowing simultaneous nitrification and denitrification. Therefore, this study provides one way to achieve simultaneous nitrification and denitrification quickly and stably. It is to be noted that the layers were formed in different periods and that this made the structure more stable, avoiding the shortcomings of the conventional one-step startup biofilm, such as instability and easy dropping off. For the two-step startup strategies, there is no need to increase the influent NH4+-N or COD loading gradually. Our results show that nitrifying bacteria can grow by apposition after seven days. The ammonia loading could be increased up to 5.4 ± 0.5 g N per m2 per d. Therefore, the startup period was shortened because of the decrease of the growth period of the slow-growing nitrifying bacteria. At the same time, there is no need to decrease the COD loading. The growth and development of heterotrophic denitrifying bacteria could be enhanced by higher COD loading. The reactor could be used for the treatment of raw wastewater within a short time. Therefore, the two-step startup strategies shorten the startup time significantly.
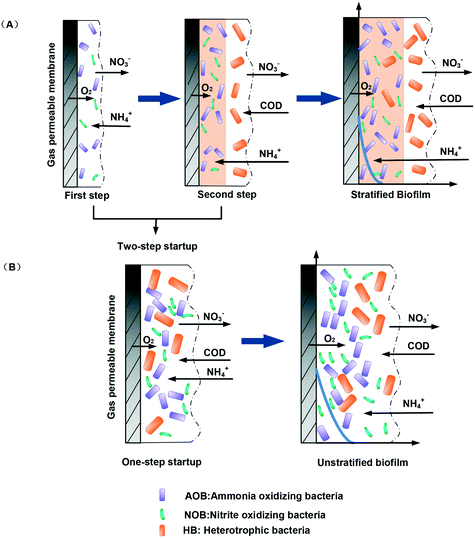 | ||
| Fig. 7 Comparison of biofilm development and structure between MABRs with the two-step startup (A) and one-step startup (B). | ||
Microbial activity measured in batch reactors using biomass extracted from MABRs R2 and R4 (Table 1) was consistent with the results of the biofilm reactors (Fig. 3 for R2 and Fig. 5 for R4). We compared the nitrification potentials estimated for batch reactors using biomass from MABRs (R2 and R4) with the ammonia removal efficiencies in the same biofilm reactors. The biomass had high nitrification potentials, SAOR 0.27 ± 0.01 g N per g SS per d for R2 and 0.36 ± 0.01 g N per g SS per d for R4 (Table 3). The high nitrification potentials of the biomass from the reactors translated to the high ammonia removal efficiencies in the MABRs (Fig. 3 for R2 and Fig. 5 for R4). The SRR for ammonia was 4.62 ± 0.48 g N per m2 per d in R2 and 5.28 ± 0.52 g N per m2 per d in R4 (Table 1).
| Reactor | SAOR(g N per g SS per d) | SDNR(g N per g SS per d) |
|---|---|---|
| Biofilm was sampled on day 63. SAOR, specific ammonium oxidation rate; SDNR, specific denitrification rate. Both R2 and R4 were operated with a two-step startup with the first step with influent containing only NH4+-N but without COD, and the second step with influent containing both NH4+-N and COD. R2 was inoculated with typical nitrifying activated sludge exposed to a low concentration of ammonium (Inoculum A) and R4 was inoculated with the acclimated biofilm from MABR fed with strong ammonium wastewater (Inoculum B). | ||
| R2 | 0.27 ± 0.01 | 0.39 ± 0.01 |
| R4 | 0.36 ± 0.01 | 0.47 ± 0.02 |
The high specific denitrification rates (SDNRs) estimated for the batch reactors indicate a high potential of denitrification in the MABRs. The SDNR for the biomass from R2 was 0.39 ± 0.01 g NO3−-N per g SS per d and that for the biomass from R4 was 0.47 ± 0.02 g NO3−-N per g SS per d. The rates of nitrogen removal by denitrification exceeded the rates of nitrogen conversion by nitrification in biomass from both R2 and R4, indicating the possibility of simultaneous nitrification and denitrification in these reactors. However, the SSRs for total nitrogen in R2 and R4, shown in Table 1, are very modest, 0.13 ± 0.53 g N per m2 per d in R2 and 1.21 ± 0.34 g N per m2 per d in R4, both much lower than the SSR for ammonium removal in these reactors, 4.62 ± 0.48 g N per m2 per d in R2 and 5.28 ± 0.52 g N per m2 per d in R4. This is not surprising. Achieving stable denitrification in MABRs treating high nitrogen and COD wastewater may be difficult because of the necessity of precise control of the oxygen flux to the biofilm. To accomplish simultaneous nitrification and denitrification, oxygen should totally penetrate the zone occupied by the nitrifiers, near the membrane, and be absent in at least part of the zone occupied by the denitrifiers, away from the membrane.12 Since heterotrophs are active in two reactions, oxidizing COD to reduce oxygen and nitrate/nitrite, to achieve simultaneous removal of COD, nitrification and denitrification, the position of the interface between the aerobic and the anoxic layers of the biofilm would have to be precisely controlled in real time. This might be accomplished with the use of stationary microsensors embedded in the biofilm and connected to a feedback control for oxygen delivery through the membrane. Without such control, the removal of nitrogen by simultaneous nitrification and denitrification may be unstable, which is in accordance with results reported in the literature.24,43,44
We believe that the two-step startup strategy of biofilm deposition in MABRs will constitute the foundation for future attempts at simultaneous nitrification and denitrification because it allows the spatial separation of nitrifiers and heterotrophs. In the future, we intend to measure microprofiles of DO, ammonia, nitrite and nitrate in MABRs put into operation using the two-step startup strategy and assess the possibility of running simultaneous nitrification and denitrification in such reactors more accurately. For now, higher rates of nitrification and COD removal in MABRs are entirely possible.
5. Conclusions
The hypothesis guiding the study was that the performance of membrane-aerated biofilm reactors (MABRs) treating strong nitrogenous and COD wastewater can be improved by implementing a startup strategy specifically designed to promote establishing a biofilm structure composed of layers of selected groups of microorganisms. According to the results of experiments we conducted to test that hypothesis, we have concluded that:(1) MABRs using the two-step startup procedure more than doubled the specific rates of ammonia and COD removal compared to reactors introduced using a one-step procedure. The specific ammonia removal rates were 5.34 ± 0.23 g N per m2 per d in the MABR with the two-step startup (R5) and 2.15 ± 0.39 g N per m2 per d in the MABR with the one-step startup (R1), and the specific COD removal rates were 12.85 ± 0.62 g COD per m2 per d in the MABR with the two-step startup (R5) and 4.78 ± 0.41 g COD per m2 per d in the MABR with the one-step startup (R1).
(2) Direct use of biomass inoculum exposed to a high concentration of ammonium shortened the startup time in comparison to inoculation with nitrifying activated sludge exposed to a low concentration of ammonium. There is no urgent need to increase either the influent NH4+-N or the COD concentration gradually during the two-step startup.
(3) Achieving stable denitrification in MABRs treating high-strength ammonia and COD wastewater may be difficult due to the challenge of precise control of oxygen flux to the biofilm, even if abundance of denitrifiers in the biofilm is not a limiting factor.
List of symbols
| AOB | Ammonia-oxidizing bacteria |
| COD | Chemical oxygen demand (mg L−1) |
| HRT | Hydraulic retention time (d) |
| MABR | Membrane-aerated biofilm reactor |
| NOB | Nitrite-oxidizing bacteria |
| SAOR | Specific ammonium oxidation rate, g NH4+-N per g SS per d |
| SDNR | Specific denitrification rate, g NO3−-N per g SS per d |
| SRR | Specific removal rate, g m−2 d−1 |
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (51878466), the National Key R&D Program of China (2016YFC0400805) and the National Science and Technology Major Project of China on Water Pollution Control and Management (2017ZX07206-001). We are grateful for support from the 111 project (B13017) of Tongji University. Dr. Rongchang Wang was supported by the Shanghai Peak Discipline Program at the Shanghai Institute of Pollution Control and Ecological Security.References
- J. G. Liu and J. Diamond, China's environment in a globalizing world, Nature, 2005, 435, 1179–1186 CrossRef CAS PubMed.
- L. K. Wang, Y. T. Hung, H. H. Lo and C. Yapijakis, Waste treatment in the process industries, CRC Press, Taylor & Francis, 2005 Search PubMed.
- A. Asadi, A. A. Zinatizadeh, L. M. Van and H. Younesiii, Nitrogen removal by ANAMMOX and simultaneous nitrification-denitrification (SND) processes in a novel single airlift bioreactor, RSC Adv., 2016, 6, 74367–74371 RSC.
- S. S. Adav, D. J. Lee and J. Y. Lai, Biological nitrification-denitrification with alternating oxic and anoxic operations using aerobic granules, Appl. Microbiol. Biotechnol., 2009, 84, 1181–1189 CrossRef CAS PubMed.
- P. A. Wilderer, J. Brautigam and I. Sekoulov, Application of gas permeable membranes for auxiliary oxygenation of sequencing batch reactors, Conserv. Recycl., 1985, 8, 181–192 CrossRef CAS.
- K. Brindle, T. Stephenson and M. J. Semmens, Pilot-plant treatment of a high-strength brewery wastewater using a membrane-aeration bioreactor, Water Environ. Res., 1999, 71, 1197–1204 CrossRef CAS.
- E. Casey, B. Glennon and G. Hamer, Review of membrane aerated biofilm reactors, Resour., Conserv. Recycl., 1999, 27, 203–215 CrossRef.
- R. C. Wang, A. Terada, S. Lackner, B. F. Smets, M. Henze, S. Q. Xia and J. F. Zhao, Nitritation performance and biofilm development of co- and counter-diffusion biofilm reactors: Modeling and experimental comparison, Water Res., 2009, 43, 2699–2709 CrossRef CAS PubMed.
- A. Terada, S. Lackner, S. Tsuneda and B. F. Smets, Redox-stratification controlled biofilm (ReSCoBi) for completely autotrophic nitrogen removal: The effect of co- versus counter-diffusion on reactor performance, Biotechnol. Bioeng., 2007, 97, 40–51 CrossRef CAS PubMed.
- R. Nerenberg, The membrane-biofilm reactor (MBfR) as a counter-diffusional biofilm process, Curr. Opin. Biotechnol., 2016, 38, 131–136 CrossRef CAS PubMed.
- E. Syron and E. Casey, Membrane-aerated biofilms for high rate biotreatment: Performance appraisal, engineering principles, scale-up, and development requirements, Environ. Sci. Technol., 2008, 42, 1833–1844 CrossRef CAS PubMed.
- R. C. Wang, F. Xiao, Y. N. Wang and Z. Lewandowski, Determining the optimal transmembrane gas pressure for nitrification in membrane-aerated biofilm reactors based on oxygen profile analysis, Appl. Microbiol. Biotechnol., 2016, 100, 7699–7711 CrossRef CAS PubMed.
- T. Ahmed, M. J. Semmens and M. A. Voss, Oxygen transfer characteristics of hollow-fiber, composite membranes, Adv. Environ. Res., 2004, 8, 637–646 CrossRef CAS.
- E. Casey, B. Glennon and G. Hamer, Oxygen mass transfer characteristics in a membrane-aerated biofilm reactor, Biotechnol. Bioeng., 1999, 62, 183–192 CrossRef CAS PubMed.
- E. Syron, M. J. Semmens and E. Casey, Performance analysis of a pilot-scale membrane aerated biofilm reactor for the treatment of landfill leachate, Chem. Eng. J., 2015, 273, 120–129 CrossRef CAS.
- E. Metcalf, Wastewater Engineering, Treatment and Reuse, 4th edn, McGraw-Hill, Boston, 2004 Search PubMed.
- M. Pankhania, K. Brindle and T. Stephenson, Membrane aeration bioreactors for wastewater treatment: completely mixed and plug-flow operation, Chem. Eng. J., 1999, 73, 131–136 CrossRef CAS.
- M. J. Semmens, K. Dahm, J. Shanahan and A. Christianson, COD and nitrogen removal by biofilms growing on gas permeable membranes, Water Res., 2003, 37, 4343–4350 CrossRef CAS PubMed.
- D. L. Timberlake, S. E. Strand and K. J. Williamson, Combined aerobic heterotrophic oxidation, nitrification and denitrification in a permeable-support biofilm, Water Res., 1988, 22, 1513–1517 CrossRef CAS.
- A. Terada, K. Hibiya, J. Nagai, S. Tsuneda and A. Hirata, Nitrogen removal characteristics and biofilm analysis of a membrane-aerated biofilm reactor applicable to high-strength nitrogenous wastewater treatment, J. Biosci. Bioeng., 2003, 95, 170–178 CrossRef CAS PubMed.
- S. Matsumoto, A. Terada, Y. Aoi, S. Tsuneda, E. Alpkvist, C. Picioreanu and M. C. M. van Loosdrecht, Experimental and simulation analysis of community structure of nitrifying bacteria in a membrane-aerated biofilm, Water Sci. Technol., 2007, 55, 283–290 CrossRef CAS PubMed.
- A. Terada, T. Yamamoto, S. Tsuneda and A. Hirata, Sequencing batch membrane biofilm reactor for simultaneous nitrogen and phosphorus removal: Novel application of membrane-aerated biofilm, Biotechnol. Bioeng., 2006, 94, 730–739 CrossRef CAS PubMed.
- L. S. Downing and R. Nerenberg, Performance and microbial ecology of the hybrid membrane biofilm process for concurrent nitrification and denitrification of wastewater, Water Sci. Technol., 2007, 55, 355–362 CrossRef CAS PubMed.
- L. S. Downing and R. Nerenberg, Total nitrogen removal in a hybrid, membrane-aerated activated sludge process, Water Res., 2008, 42, 3697–3708 CrossRef CAS PubMed.
- S. Lackner, A. Terada and B. F. Smets, Heterotrophic activity compromises autotrophic nitrogen removal in membrane-aerated biofilms: Results of a modeling study, Water Res., 2008, 42, 1102–1112 CrossRef CAS PubMed.
- C. Pellicer-Nacher, S. P. Sun, S. Lackner, A. Terada, F. Schreiber, Q. Zhou and B. F. Smets, Sequential aeration of membrane-aerated biofilm reactors for high-rate autotrophic nitrogen removal: experimental demonstration, Environ. Sci. Technol., 2010, 44, 7628–7634 CrossRef CAS PubMed.
- K. R. Gilmore, A. Terada, B. F. Smets, N. G. Love and J. L. Garland, Autotrophic nitrogen removal in a membrane-aerated biofilm reactor under continuous aeration: A demonstration, Environ. Eng. Sci., 2013, 30, 38–45 CrossRef CAS.
- L. S. Downing and R. Nerenberg, Effect of bulk liquid BOD concentration on activity and microbial community structure of a nitrifying, membrane-aerated biofilm, Appl. Microbiol. Biotechnol., 2008, 81, 153–162 CrossRef CAS PubMed.
- T. M. LaPara, A. C. Cole, J. W. Shanahan and M. J. Semmens, The effects of organic carbon, ammoniacal-nitrogen, and oxygen partial pressure on the stratification of membrane-aerated biofilms, J. Ind. Microbiol. Biotechnol., 2006, 33, 315–323 CrossRef CAS PubMed.
- Y. J. Feng, S. K. Tseng, T. H. Hsia, C. M. Ho and W. P. Chou, Partial nitrification of ammonium-rich wastewater as pretreatment for anaerobic ammonium oxidation (Anammox) using membrane aeration Bioreactor, J. Biosci. Bioeng., 2007, 104, 182–187 CrossRef CAS PubMed.
- K. Pynaert, B. F. Smets, D. Beheydt and W. Verstraete, Start-up of autotrophic nitrogen removal reactors via sequential biocatalyst addition, Environ. Sci. Technol., 2004, 38, 1228–1235 CrossRef CAS PubMed.
- A. Schramm, L. H. Larsen, N. P. Revsbech, N. B. Ramsing, R. Amann and K. H. Schleifer, Structure and function of a nitrifying biofilm as determined by in situ hybridization and the use of microelectrodes, Appl. Environ. Microbiol., 1996, 62, 4641–4647 CAS.
- T. G. Li, J. X. Liu, R. B. Bai and F. S. Wong, Membrane-aerated biofilm reactor for the treatment of acetonitrile wastewater, Environ. Sci. Technol., 2008, 42, 2099–2104 CrossRef CAS PubMed.
- J. H. Rotthauwe, K. P. Witzel and W. Liesack, The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations, Appl. Environ. Microbiol., 1997, 63, 4704–4712 CAS.
- H. M. Dionisi, A. C. Layton and G. Harms, Quantification of nitrosomonas oligotropha-like ammonia-oxidizing bacteria and nitrospira spp. from full-scale wastewater treatment plants by competitive PCR, Appl. Environ. Microbiol., 2002, 68, 245–253 CrossRef CAS PubMed.
- D. Bru, A. Sarr and L. Philippot, Relative abundances of proteobacterial membrane-bound and periplasmic nitrate reductases in selected environments, Appl. Environ. Microbiol., 2007, 73, 5971–5974 CrossRef CAS PubMed.
- C. López-Gutiérrez, S. Henry and S. Hallet, Quantification of a novel group of nitrate-reducing bacteria in the environment by real-time PCR, J. Microbiol. Methods, 2004, 57, 399–407 CrossRef PubMed.
- T. Yan, M. W. Fields, L. Wu, Y. Zu, J. M. Tiedje and J. Zhou, Molecular diversity and characterization of nitrite reductase gene fragments (nirK and nirS) from nitrate- and uranium-contaminated groundwater, Environ. Microbiol., 2003, 5, 13–24 CrossRef CAS PubMed.
- J. Geets, M. de Cooman, L. Wittebolle, K. Heylen, B. Vanparys, P. De Vos, W. Verstraete and N. Boon, Real-time PCR assay for the simultaneous quantification of nitrifying and denitrifying bacteria in activated sludge, Appl. Microbiol. Biotechnol., 2007, 75, 211–221 CrossRef CAS PubMed.
- H. L. Tian, J. Y. Zhao, H. Y. Zhang, C. Q. Chi, B. A. Li and X. L. Wu, Bacterial community shift along with the changes in operational conditions in a membrane-aerated biofilm reactor, Appl. Microbiol. Biotechnol., 2015, 99, 3279–3290 CrossRef CAS PubMed.
- R. C. Wang, X. M. Zhan, Y. L. Zhang and J. L. Zhao, Nitrifying population dynamics in a redox stratified membrane biofilm reactor (RSMBR) for treating ammonium-rich wastewater, Front. Environ. Sci. Eng., 2011, 5, 48–56 CrossRef CAS.
- L. S. Downing and R. Nerenberg, Effect of oxygen gradients on the activity and microbial community structure of a nitrifying, membrane-aerated biofilm, Biotechnol. Bioeng., 2008, 101, 1193–1204 CrossRef CAS PubMed.
- K. Hibiya, A. Terada, S. Tsuneda and A. Hirata, Simultaneous nitrification and denitrification by controlling vertical and horizontal microenvironment in a membrane-aerated biofilm reactor, J. Biotechnol., 2003, 100, 23–32 CrossRef CAS PubMed.
- A. Kumar, A. Hille-Reichel, H. Horn, J. Dewulf, P. Lens and H. Van Langenhove, Oxygen transport within the biofilm matrix of a membrane biofilm reactor treating gaseous toluene, J. Chem. Technol. Biotechnol., 2012, 87, 751–757 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |