 Open Access Article
Open Access ArticleEngineered nanomaterials in the context of global element cycles†
Nina Z.
Janković
 ac and
Desirée L.
Plata
ac and
Desirée L.
Plata
 *abc
*abc
aDepartment of Chemical and Environmental Engineering, Yale University, New Haven, CT 06511, USA
bDepartment of Civil and Environmental Engineering, Massachusetts Institute of Technology, Cambridge, MA 02142, USA. E-mail: dplata@mit.edu
cCenter for Green Chemistry and Green Engineering, Yale University, 225 Prospect Street, New Haven, CT 06520, USA
First published on 23rd July 2019
Abstract
Environmental nanomaterials researchers are challenged to discern relevant use and release scenarios of engineered nanomaterials (ENMs). Here, we evaluated ENMs within the framework of global anthropogenic element cycles. To provide a bird's-eye view of the status and scale of nanotechnologies, we constructed a multifaceted framework to discern industrial relevance by employing metrics, such as technology readiness level, annual production volumes, synthetic efficiencies, and projected annual market growth rates across twenty-five ENMs. For eight detailed element cycles (Ce, Ag, Zn, Al, Co, Cu, Ni, and Fe), ENMs had a minor influence on anthropogenic element cycling (2 × 10−6 to 2% of total extracted ore), while nSiO2 represents 3–25% of Si metal mined. Production volumes represent only a portion of the material mined for nanomaterial synthesis; synthetic yields for metal, metalloid, and metal oxide nanomaterials were high (typically greater than 90%), while carbon-based nanomaterials have dramatically lower synthetic efficiencies (8–33%). Finally, technology readiness levels indicated that carbon-based nanomaterials have a diverse suite of current applications, whereas metal and metalloid-oxide applications are more limited in number. Several markets continue to grow, particularly quantum dots (58% projected annual growth from 2015–2025). Probing the vast nanomaterial space en masse serves to focus environmental health and safety efforts on materials that are most industrially relevant to biogeochemical processes, and this article is first to consider ENMs within the framework of anthropogenic element cycling of bulk materials at the global level.
Environmental significanceDisturbance of global element cycles can serve as a predictor of ecotoxicological outcomes, and consideration of the rates of engineered nanomaterial (ENM) element mobilizations could help prioritize efforts to understand environmental risks. Here, we present market-based metrics of production volumes, readiness levels, and anticipated growth rates to guide relevant environmental health research. Findings indicate ENM-derived mobilizations are small compared to global element extractions, except in the case of nSiO2, and market analyses suggest opportunities to perform more focused and relevant EHS research. Ultimately, this geochemical modeling coupled with market analysis could provide a framework for prioritization of research efforts for all commercialized and emerging technologies. |
Introduction
Perhaps more than any other industry, nanotechnology has developed with at least some application of the precautionary principle and early assessment of anticipated environmental impacts. For example, in Europe, engineered nanomaterials (ENMs) have been regulated under the REACH initiative, which has required collection of environmental performance data prior to commercialization of ENM-enabled products (e.g., predicted or derived no-effect exposure levels for compounds produced above 10 metric tons per year and physicochemical properties, such as flash point or octanol–water partitioning coefficients for compounds produced above 1 metric ton per year).1 In the United States (US), while no such restrictions were placed on commercial applications of ENMs, the National Science Foundation (NSF) and Environmental Protection Agency (EPA) jointly sponsored two major research networks ($77.4m to date to the University of California's Center for the Environmental Implications of Nanotechnology (UC CEIN) and Duke University's Center for the Environmental Implications of Nanotechnologies (CEINT))2–5 to address questions related to the unintended implications of ENMs, their ecotoxicity, and their environmental fate. In addition, the EPA-sponsored project Lifecycle of Nanomaterials (LCNano) was aimed at understanding the relationships between nanomaterial properties and exposure or hazard, through lifecycle considerations ($5m over four years6). In spite of these notable investments, the public expenditure in such early environmental optimization has been small relative to the investment in the development of novel ENMs (e.g., from 2007 to 2017, $978.2m in nano environmental health and safety (EHS) compared to $17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820.8m total in nanomaterial funding7–9). As such, prioritization schemes are necessary10 to focus environmental health and safety research on those nanomaterials that are either rapidly emerging, have large projected market sizes, or anticipated hazard (e.g., implicit toxicity).
820.8m total in nanomaterial funding7–9). As such, prioritization schemes are necessary10 to focus environmental health and safety research on those nanomaterials that are either rapidly emerging, have large projected market sizes, or anticipated hazard (e.g., implicit toxicity).
In 1975, Garrels et al.11 reflected on the use of geochemical understanding to forecast risk. Specifically, Garrels synthesized permissible exposure limits for several ions and total dissolved solids in the United States and the average world stream concentrations, noting a positive correlation between the two. This loose correlation persists today for both US Environmental Protection Agency12 and World Health Organization13 recommendations and refined freshwater element concentrations13,14 (Fig. 1). The relationship suggests that organisms have evolved to tolerate exposure to elements in proportion to those elements' natural abundances in the environment. Relatedly, others have noted that relative element distributions in the seawater and living organisms (i.e., humans and bacteria)15 are positively correlated, and the relation between elemental abundance distributions in living organisms and natural environments are cited as support for theory on the origins of life.16 Extending this line of thought, Garrels postulated that anthropogenic disturbances in natural element cycles (i.e., via enhanced withdrawal or mobilization rates) could give rise to observed environmental malignancies or toxic responses. Many examples exist on local levels17–22 and globally for some elements, such as mercury, where human disturbances of the mercury cycle23 have led to systematic increases in oceanic concentrations of methyl mercury, a well-documented neurotoxin.24
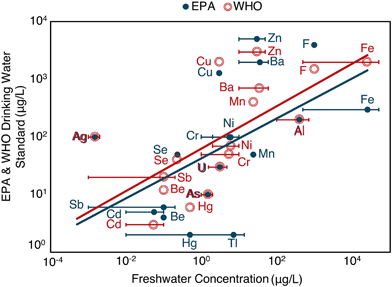 | ||
| Fig. 1 Relationship between EPA12 or WHO13 drinking water standards or regulations and freshwater elemental concentrations13,14 in μg L−1 (log–log plot). Bars represent ranges of freshwater elemental concentrations. Points represent the average of the ranges. Adapted from Garrels et al. (1975).11 | ||
The explicit toxicity of ENMs has been assessed via animal,25–28 cellular,28–32 and molecular33–35 models. Several questions remain regarding the validity of these approaches and the conclusions drawn from them for at least two major reasons: (1) the methods were developed for dissolved chemicals, and nanoparticles may not be stable in the selected test media (i.e., exposure to the ENM might be reduced or absent due to particle flocculation or coagulation), and (2) concentrations of ENMs have been extremely high in many test cases, encouraging coagulation and thereby reducing exposure. Accepting those limitations, the current literature holds that, while some engineered nanomaterial-specific toxicity mechanisms have been observed (e.g., unique bandgaps enabling generation of reactive oxygen species at ambient conditions28,36,37 or morphological influences38,39),40 several ENMs can exert toxicity through release of their corresponding ions (e.g., Ag41 and Cd42). That is, the mechanism of toxicity is not necessarily unique to the nanosize fraction, except for the influence of size on surface-area-to-volume ratios and corresponding ion dissolution rates43 and on the ability to access or be cleared from physiological compartments.42,44,45 In these cases, we note that incidental and naturally occurring analogs (i.e., incidental nanomaterials (INMs) and natural nanomaterials (NNMs) or colloids) exist and are implicit in global element cycle inventories. Thus, environmental impacts from ENMs may manifest simply through mobilization of the element and disturbance of the element cycle itself.
Proactive researchers sought to bound these mobilization rates through assessment of nanomaterial market sizes,46 modeled environmental concentrations,10,47–49 and estimation of impacted environmental compartments through an understanding of deployed nanomaterial applications.50 Here, we evaluated the influence of recent ENM production estimates on global element cycles of relevant commercial materials. Importantly, we refer readers to Klee and Graedel's seminal work (2004)51 and Sen and Peucker-Ehrenbrink's update (2012)52 delineating anthropogenic and natural mobilization rates and note that nanomaterial production may influence those anthropogenic rates. Briefly, we highlight that anthropogenic element cycles account for and quantify the stocks and flows of materials mobilized through human activity across relevant reservoirs (see ESI† glossary following Table S1), whereas natural element cycles account for material stocks, flows, and biogeochemical pathways by which elements are transformed or mobilized through various environmental compartments. A dominant tool of anthropogenic cycle assessment has been material flow analyses (MFAs), in which established system boundaries and mass balance accounting provide a route to elucidate anthropogenic cycles of many elements.53–56 In the context of these global anthropogenic element mobilizations, the objective of this work is to quantify nanomaterial-derived influences using current production volumes and anticipated market growth with the ultimate goal of identifying priority research areas, relevant release scenarios, and providing context for current nano EHS investigations.
Methods
Status and scale of nanotechnologies
In order to assess the status of developing nanotechnologies, we considered the technology readiness level (TRL) of applications that employ engineered nanomaterials based on a 2016 report by Future Markets Inc. (The Global Market for Nanotechnology and Nanomaterials).57 Here, we acknowledge that these data can only be as veracious as the reporting entities who were surveyed. Nevertheless, the data can be viewed as trustworthy estimates as both the surveyor and, if a public company, the surveyed maintain a legal obligation to deliver accurate estimates of market sizes and readiness levels to investors.The TRL metric indicates the maturity of a technology; the nine-level scale was developed by NASA in the 1980's and has subsequently been adapted to gauge innovations across a broad range of fields.58 Here, we excluded levels 1 (i.e. basic principles observed and reported) and 2 (i.e. technology concept and/or application formulated), due to the abundance of research conducted at these levels, and report condensed levels 3–9 to provide granular information on the state of development. This resulted in three levels designated applied R&D (TRLs 3 and 4), demonstration (TRLs 5–7), and commercial (TRLs 8 and 9)57 (Table 1).
| Modified TRLs as defined by Future Markets Inc. | TRL as defined by U.S. DOE | ||
|---|---|---|---|
| Note that the United States' National Nanotechnology Coordination Office (NNCO) has introduced data readiness levels (DRLs),59 which demonstrate an existing link between nanoEHS communities and the TRL concept. | |||
| Applied R&D | 3 | Proof of concept | Analytical and experimental critical function and/or characteristic proof-of-concept |
| 4 | Lab tested | Component and/or system validation in laboratory environment | |
| Demonstration | 5 | Field tested | Laboratory scale, similar system validation in relevant environment |
| 6 | Basic prototype | Engineering/pilot-scale, similar (prototypical) system validation in relevant environment | |
| 7 | Final prototype | Full-scale, similar (prototypical) system demonstrated in relevant environment | |
| Commercial | 8 | Fully tested | Actual system completed and qualified through test and demonstration |
| 9 | In operation | Actual system operated over the full range of expected conditions | |
Nanomaterial production relative to global mining rates
Global nanomaterial production volumes for 2014 were obtained from The Global Nanotechnology and Nanomaterials Market Opportunity Report (2016).57 These data were compared to reported mine production values for 2014 obtained from the U.S. Geological Survey (USGS) Minerals Yearbook and Commodity Summaries (see ESI†) and placed in the context of the material extraction efficiency across the mineral life cycle (see methods below) as a mechanism to assess the size of the nanomaterials' respective markets relative to other dominant material flows. We emphasize that global material extractions and nanomaterial production volumes are from the same year (2014) for comparative accuracy; however, global element extraction efficiencies (see description below) were determined across several years of research in the literature and then applied to the 2014 production volumes.Note that the USGS Minerals Yearbook and Commodity Summaries of certain minerals may contain data pertaining to only specific minerals when others are mined in significant volumes. Conversely, some elements have multiple dominant sources. These must be addressed to avoid over or underestimating mining rates. Specifically, we note necessary caveats in two cases: magnesium and silicon. First, the USGS summary reports magnesium mine production as magnesite only, noting that magnesium and magnesium compounds are also produced from dolomite, brucite, carnallite, olivine minerals, seawater, and well and lake brines and bitterns.60–62 To approximate the total magnesium mine production, we considered the sum of magnesite mine production and magnesium metal production, in mass of magnesium content. Second, the USGS reports total silicon mine production as a combined total of silicon metal and ferrosilicon in mass of estimated silicon content or individually in gross weight. Note that silicon metal is defined as metal containing high, but less than 99.9% silicon content, whereas ferrosilicon has variable silicon content (e.g., two standard grades include 50% and 75% silicon). Ultra-high purity grades of silicon metal (equal to or greater than 99.9% silicon content) used in semiconductor and solar industries are excluded in USGS surveys; however, those grades are derived from further processing of lower grade silicon metal (i.e., the mass fraction represented by these ultra-high purity grades is implicitly included in the silicon metal grade as defined by USGS). As silica nanomaterials are ultimately derived from silicon metal (via silicon containing precursors, such as tetraethoxysilane (TEOS)), rather than ferrosilicon or silicon ferroalloys or miscellaneous silicon alloys, we considered only the mass of silicon metal produced in 2014 and assume the silicon content to be circa 100%.63 To assess the sensitivity of our calculations to this exclusion, we provide alternative calculations (see Table S2†) where the aggregate of silicon metal and ferrosilicon production values are used to estimate global mine production.64
Global element efficiencies across the mineral life cycle
Briefly, we delineate that material element “efficiency”65 (in the context of the anthropogenic life cycle) refers to the amount of total material anthropogenically mobilized by ore extraction from the earth that is moved forward along various stages of the material life cycle. That is, the efficiencies capture the ratio of material that goes on in useful life rather than being “lost” to other compartments that might be regarded as waste, such as tailings and slag.The anthropogenic flows considered herein are those from mining, spanning the entire life cycle of a primary material from raw material extraction to the end-of-life (EOL) phase. World mine, smelter, and/or refinery production volumes for 2014 were obtained from the U.S. Geological Survey (USGS) Minerals Yearbook, USGS Commodity Summaries, and the International Nickel Study Group (INSG) (ESI†). (Note that the USGS often excludes U.S. production volumes or limits detailed information to avoid disclosing proprietary data). Starting from these global extraction values, material efficiencies across a single life cycle were calculated from global anthropogenic element cycles available in the literature (i.e., percentage efficiencies were calculated from cycling information available in the nearest year; see Table 2). Primary material flows (i.e. materials originating directly from ores) were decoupled from secondary flows (i.e. materials originating from scrap sources, EOL products, or fabrication residues), within the limit of available data, using cycling rates. Efficiencies were calculated across one life cycle (i.e., from extraction to EOL of only primary materials) and stock accumulation was omitted (i.e., total ore production volume was assumed to reach the EOL within the same year). Details of the calculations, assumptions, and exceptions are noted in the ESI.†
| Element | Cycle year | Ref. |
|---|---|---|
| Ag | 1997 | Johnson et al., 200566 |
| 2014 | U.S. Geological Survey Minerals Yearbook 201467 | |
| Al | 2009 | Liu et al., 201268 |
| 2014 | U.S. Geological Survey Minerals Yearbook 201569 | |
| Ce | 2007 | Du and Graedel, 201170 |
| 2014 | U.S. Geological Survey Minerals Yearbook 201471 | |
| Co | 2005 | Harper et al., 201272 |
| 2014 | U.S. Geological Survey Minerals Yearbook 201473 | |
| Cu | 2013 | Glöser et al., 201374 |
| International Copper Study Group, 201576 | ||
| 2014 | U.S. Geological Survey Minerals Yearbook 201475 | |
| Fe | 2000 | Wang et al., 200777 |
| 2014 | U.S. Geological Survey Minerals Yearbook 2014- Advance Data Release78 | |
| Ni | 2005 | Reck and Graedel, 201265 |
| 2014 | U.S. Geological Survey Mineral Commodity Summaries 201679 | |
| International Nickel Study Group, World Nickel Statistics 201580 | ||
| Zn | 2010 | Meylan and Reck, 201581 |
| 2014 | U.S. Geological Survey Minerals Yearbook 201482 |
Nanomaterial production and market growth rates
Nanomaterial market production (2010–2015) and projected market production (2015–2025) growth rates were calculated according to the annual growth rate equation: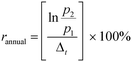 | (1) |
Results and discussion
Environmental nanomaterials researchers are challenged to discern relevant use and release scenarios for specific applications.50,86 Indeed, a large determinant of early nanoEHS investigations was a balance of existing characterization, detection analytical capabilities, and understanding of emergent applications at the time. Today, semi-quantitative knowledge of current and developing nanotechnologies, as well as production volumes (Fig. 2), are invaluable to prioritization of EHS research. In the absence of a single dominant use, it is not obvious which potential release scenarios are worthy of evaluation or if there is a dominant application at all. Indeed, the lack of a prevailing technology could create a false perception that the materials are not used in large quantities and, accordingly, lack substantial environmental distribution. A number of ENMs have potential for high applications diversity (e.g., graphene and CNTs) and may have diverse exposure routes. For example, carbon nanotubes (CNTs) do not have a single “killer application” (i.e., dominant use for which the material is known), but they are widely used for a variety of commercialized purposes (n = 16; see ESI† or an enlarged Fig. 2a), and an estimated 550–2750 Mg (metric tons) were produced in 2015. To put this number into context, at the height of the polychlorinated biphenyl (PCB) industry, annual production was on the order of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons,87 while the global market for polytetrafluorethylene (PTFE) was 165
000 metric tons,87 while the global market for polytetrafluorethylene (PTFE) was 165![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons88 in 2015. Thus, the high-volume ENMs are within an order of magnitude of some large-volume industrial chemicals. In contrast with many bulk chemicals, some nanomaterials have a large diversity of uses. A large number of applications indicates the potential to influence numerous markets and become widely distributed in a variety of consumer goods (i.e., there would be a suite of potential exposure routes). Of course, while some nanomaterial markets are diverse, there are counter examples: some nanomaterials have only a single application currently, such as Bi2O3 (used for ceramics and produced at an estimated 37–67 Mg in 2015) or antimony tin oxide (ATO; used as antistatic coatings and produced at an estimated 130–255 Mg in 2015). Production volumes of other nanomaterials ranged from 0.37–1.48 Mg (dendrimers) to 210
000 metric tons88 in 2015. Thus, the high-volume ENMs are within an order of magnitude of some large-volume industrial chemicals. In contrast with many bulk chemicals, some nanomaterials have a large diversity of uses. A large number of applications indicates the potential to influence numerous markets and become widely distributed in a variety of consumer goods (i.e., there would be a suite of potential exposure routes). Of course, while some nanomaterial markets are diverse, there are counter examples: some nanomaterials have only a single application currently, such as Bi2O3 (used for ceramics and produced at an estimated 37–67 Mg in 2015) or antimony tin oxide (ATO; used as antistatic coatings and produced at an estimated 130–255 Mg in 2015). Production volumes of other nanomaterials ranged from 0.37–1.48 Mg (dendrimers) to 210![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–1
000–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600
600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Mg (SiO2) in 2015, with SiO2, TiO2, clays, ZrO2, ZnO, Al2O3, and CNTs representing the largest markets by mass. Interestingly, some of the nanomaterials that have been the subject of a great deal of EHS research (see Fig. S1†), such as Ag, iron oxides, fullerene, and Au nanoparticles, represent relatively small current markets (150–450 Mg, 9.2–54 Mg, 50–115 Mg, and 1.3–3.1 Mg, respectively in 2015). Here, we emphasize that early safety research should not depend on market size only, but can be aided by understanding relevant applications, use scenarios, and corresponding environmental mobilizations.
000 Mg (SiO2) in 2015, with SiO2, TiO2, clays, ZrO2, ZnO, Al2O3, and CNTs representing the largest markets by mass. Interestingly, some of the nanomaterials that have been the subject of a great deal of EHS research (see Fig. S1†), such as Ag, iron oxides, fullerene, and Au nanoparticles, represent relatively small current markets (150–450 Mg, 9.2–54 Mg, 50–115 Mg, and 1.3–3.1 Mg, respectively in 2015). Here, we emphasize that early safety research should not depend on market size only, but can be aided by understanding relevant applications, use scenarios, and corresponding environmental mobilizations.
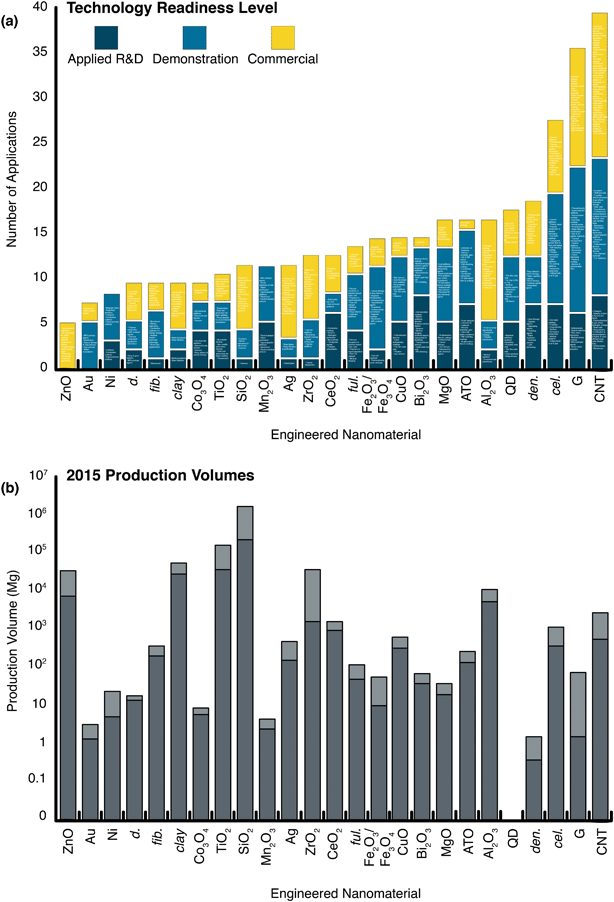 | ||
| Fig. 2 The status and scale of nanotechnologies. (a) Specific applications and/or application categories are listed (enlarge to view or see Table S3 ESI†) within the following technology readiness levels: applied R&D: proof of concept and/or lab tested; demonstration: field tested, basic prototype, and/or final prototype; commercial: fully tested and/or in operation. (b) Global production volume estimates for 2015 (log scale). The quantum dot (QD) market was not reported on a mass basis, apart from 2016 and 2020 gross estimates (refer to Table S6†); the global quantum dot revenue for the year 2015 was $400–600m. d.: diamonds, fib.: fibers, clay: clays, ful.: fullerenes, ATO: antimony tin oxide (SnO2/Sb2O5), QD: quantum dot, den.: dendrimers, cel.: cellulose, G: graphene, CNT: carbon nanotubes, UV: ultraviolet, MRI: magnetic resonance imaging, LIB: lithium-ion batteries, RFI: radio frequency interference, IR: infrared radiation, SIB: sodium-ion batteries, EMI: electromagnetic interference, LCD: liquid crystal display, TV: television, 3D: three-dimensional, TCF: transparent conductive film, PEM: polymer electrolyte membrane/proton exchange membrane, E-textiles: electronic textiles, ESD: electrostatic discharge, TEM: transmission electron microscopy, SPM: scanning probe microscopy, AFM: atomic force microscopy. Data sourced from Future Markets, Inc., The Global Nanotechnology and Nanomaterials Market Opportunity Report, 2016.57 | ||
Probing the nanomaterial space en masse, we find ENM-enabled technologies are in various stages of maturity. Among the larger markets, the current realized applications for the metal oxides tend to be fewer in number than carbon-based nanomaterials: the ZnO and TiO2 nanotechnologies spaces are dominated by cosmetics (85 and 72%, respectively), followed by coatings (12% and 20%, respectively), and a handful of other small applications, whereas graphene and carbon nanotubes have no clearly dominant uses (e.g., “electronics,” “energy,” and “composites” occupied around 13, 15, and 18% of the total CNT market in 2015) (Fig. 3). SiO2, the largest nanomaterial produced by mass, has a fairly even distribution of applications in robust markets (e.g., composites and reinforcements (38%), paints and coatings (25%), adhesives (15%), and electronics (12%)). Note that some of these uses have much higher anticipated or inherent release rates and/or distinct exposure routes, such as the paints, coatings, and cosmetic functions typical of the majority of metal oxide uses (see the work of the Keller group for anticipated releases and fates50,86). Pure metals and metal oxides tend to have fewer developed and developing nanotechnologies in the applied R&D phases (Fig. 2), with the exception of quantum dots (QDs). QDs have roughly 5–7 applications in each of the TRL categories, and this diversity may contribute to the large anticipated market growth (0.5 to 5.0 Mg per year global production projected between 2016 and 2020). Nevertheless, in spite of several recently realized commercial deployments (e.g., quantum dot LED (light-emitting diode) backlit liquid crystal displays (LCDs) and medical contrast agents), current QD markets are trivial by mass, a consequence of the fact that applications require small masses of material (e.g., on the order of kg per year for the entire LCD QD market89). Note that these applications are dominated by CdTe and CdSe (both with implicit toxicity14,90), which have few natural analogs and a small number of other anthropogenic uses. As a result, elements that were previously rare in consumer goods (i.e., Cd and Se) become widely distributed via the production, use, and disposal of the relevant products. Further, in the case of QDs, the nanoform may be a unique vector for transport of toxic elements, but nevertheless illustrates the value in contextualizing exposure with other relevant geochemical sources of those materials. For these and all growing nanomaterial markets, the question arises as to whether the nanomaterial production itself dominates or will ever dominate over the other element mobilizations.
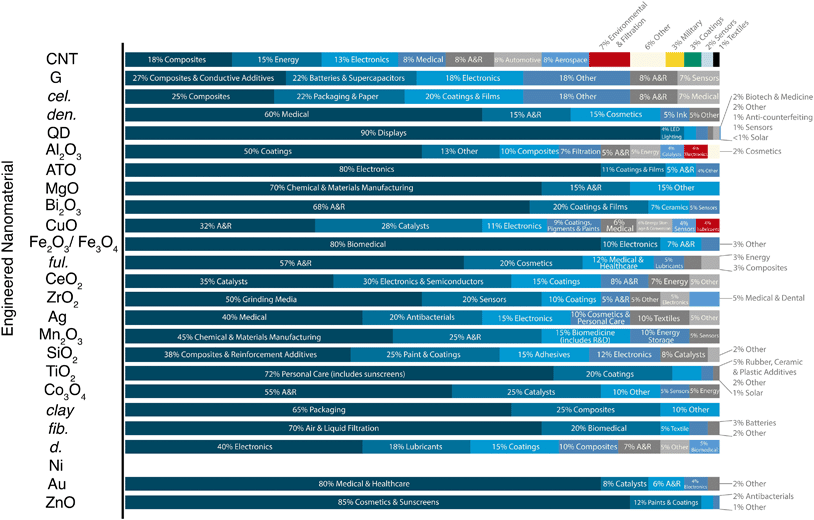 | ||
| Fig. 3 Engineered nanomaterial (ENM) market demand by application category. A&R: academia and research. ENM abbreviations are defined in Fig. 2. | ||
Klee and Graedel (2004)51 built the foundation for this and related questions using anthropogenic (i.e., industrial and incidental) and natural element (i.e., undisturbed biogeochemical) cycle information available at the end of the 20th century. Sen and Peucker-Ehrenbrink (2012)52 updated and added an accounting of human's influence on soil erosion and aeolian dust flux and the associated, indirect but nevertheless anthropogenic, mobilization of elements. Remarkably, the human contribution was more than 50% of all soil erosion and aeolian dust for all 77 queried elements. Of those, anthropogenic mobilization outweighed natural cycling for 26 elements, including Cu, Fe, Ag, Sn, As, Au, Sb, Bi, Fe, Ag, Sn, Ni, Zn, Cu, and In, which have emergent and established nanotechnologies. Natural mobilization dominated over the anthropogenic mobilization for 51 elements, including the nano-relevant elements such as Cd, Mg, Mn, Al, Se, Co, C, and Si, even considering that the latter two have extremely large industrial uses in non-nano applications (e.g., activated carbons, diamond, rubbers, glasses, ceramics, and semiconducting quartz). In this work, we determined the influence of nanomaterials on those global biogeochemical cycles to highlight areas where anthropogenic, nano-related element mobilization may make measurable contributions.
Armed with basic knowledge of nanomaterial market sizes and mining rates, it is clear that nanomaterial applications are not a major driver of mining activities (i.e., the nanomaterial markets are relatively small compared to all other industrial uses for any given element). Thus, the volume of material extracted for engineered nanomaterial production is better contextualized by the relative size of fractional losses and uses across the anthropogenic material cycles. Modifying the approach of Reck and Graedel (2012),65 we overlaid nanomaterial production within global anthropogenic efficiencies (Fig. 4), considering three types of losses from extraction to EOL: (1) tailings and slag, (2) landfill, and (3) remaining dominant categories (e.g., in-use dissipation, separation and losses, fabrication and manufacturing losses, or non-functional recycling). Generally speaking, nanomaterial uses are always minor compared to other fractions, even when they are substantial (e.g., on the order of full percentages of the total mining), and they are always at least one order of magnitude smaller than losses to tailings and slag. (Note that the size fraction of particles in tailings and slag tends to be on the order of 100 nm to 300 μm;91,92 these are microscale particles and do not represent a major source of incidental nanoparticles). The largest proportions of nanomaterial mobilization are nano-TiO2 (0.5–2% nano of 3620 Gg mined), nano-Zr (0.2–4% nano of 671 Gg mined), and nano-Si (3–25% nano of 2600 Gg metal mined) (Table 3. Note that these elements are omitted from Fig. 4 due to a lack of specific information on the anthropogenic life cycle efficiencies, such as the fraction associated with tailings and slag or recycling (see ESI†). Nevertheless, the total mined material is well known, allowing for a calculation of the nanomaterial market fraction). The markets for nanoscale cerium, silver, and zinc also represent a notable, but nevertheless small, fraction of the use phase, at 2% (1 Gg relative to 63.6 Gg extracted), 1% (0.4 Gg relative to 33.4 Gg extracted), and 0.2% (26 Gg relative to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 Gg extracted), respectively (Fig. 4). The unifying feature of these elements are that the nanomaterial market is relatively large while the anthropogenic mobilization for non-nano applications is somewhat modest. In contrast, the smallest nanomaterial contributions to element cycling occur where there are modest nanomaterial markets but very large non-nano industrial demands for elements. This is the case for copper at 0.002% (0.5 Gg relative to 22
300 Gg extracted), respectively (Fig. 4). The unifying feature of these elements are that the nanomaterial market is relatively large while the anthropogenic mobilization for non-nano applications is somewhat modest. In contrast, the smallest nanomaterial contributions to element cycling occur where there are modest nanomaterial markets but very large non-nano industrial demands for elements. This is the case for copper at 0.002% (0.5 Gg relative to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 Gg extracted), cobalt at 0.003% (0.006 Gg relative to 246 Gg extracted), nickel at 0.007% (0.02 Gg relative to 2810 Gg extracted), aluminum at 0.008% (5 Gg relative to 67
400 Gg extracted), cobalt at 0.003% (0.006 Gg relative to 246 Gg extracted), nickel at 0.007% (0.02 Gg relative to 2810 Gg extracted), aluminum at 0.008% (5 Gg relative to 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 Gg extracted), and iron at 0.000002% (0.03 Gg relative to a staggering 1
600 Gg extracted), and iron at 0.000002% (0.03 Gg relative to a staggering 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650
650![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Gg extracted).
000 Gg extracted).
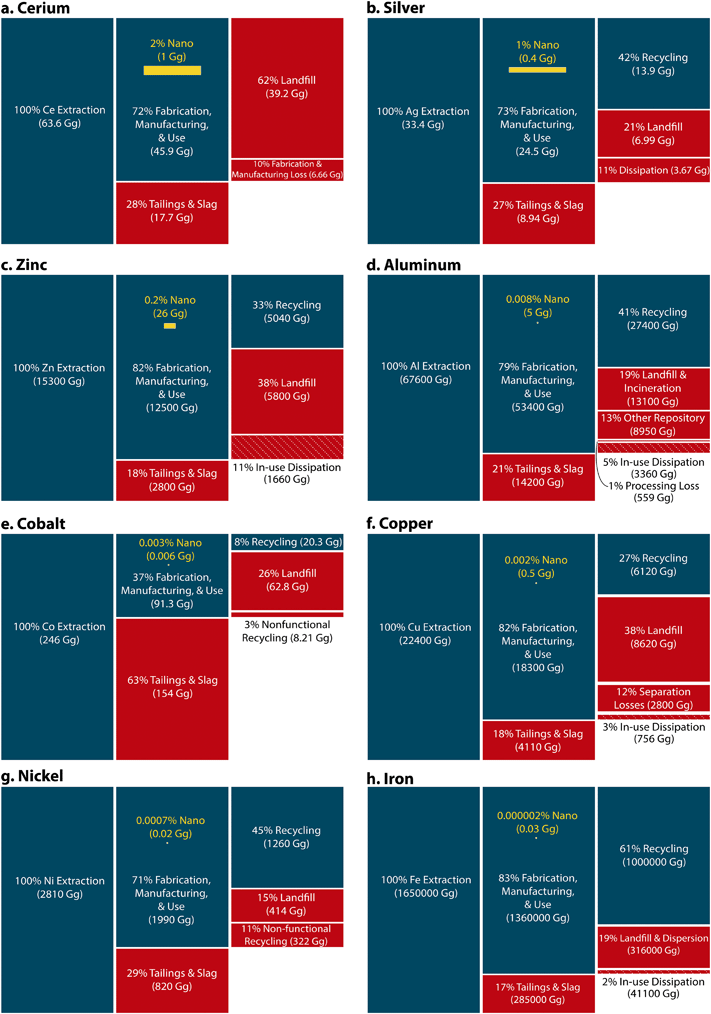 | ||
| Fig. 4 Global material efficiencies across one life cycle of the particular element, from extraction of ore to end-of-life (left to right): production, fabrication, manufacturing, and use, and waste management/end-of-life. Red: losses from the anthropogenic cycle, where stripped areas are losses during the use phase; blue: flows of metal to the anthropogenic cycle; yellow: engineered nanomaterial. Areas of rectangles reflect the magnitude of material flows as the percent of extracted ore in Gg of metal content, based on 2014 production volumes. Nanomaterial flows are based on high production volume estimates for 2014. All masses are in metal content of the particular element. All masses are rounded to 3 significant digits with the exception of nanomaterial flows. A glossary of terms is provided in the ESI† Table S1. | ||
| Nano-element | Fraction of mine production 2014a (%) | Percent yieldb (ca.) | |
|---|---|---|---|
| a Percent global ENM production (low to high estimates) of global mine production with the exception of Al and Si (which are reported relative to smelter production). Total magnesium production includes production of metallic magnesium. b Fabrication efficiencies for various nanomaterials reported in the literature are shown. Example efficiencies available in the literature may not represent industrial efficiencies. High: high yield. c Antimony tin oxide efficiency shown. d Cobalt hydroxide efficiency (cobalt oxide is readily prepared by thermal decomposition from cobalt hydroxide). e Efficiency of combustion product MgO (ca. 100%). f Mg(OH)2 efficiency (ca. 95%). g Efficiency of precursor [Mn(BTO)(H2O)2]n. h Few layer graphene | |||
| Natural flows dominate element mobilization | Ag | 0.5–2 | 100%,93 high94,95 |
| Au | 0.04–0.1 | 90–99%,96 high94 | |
| Bi | 0.3–0.5 | 99%97 | |
| Ce | 1–2 | 100%98,99 | |
| Cu | 0.001–0.002 | 100%100,101 | |
| Fe | 4 × 10−7–2 × 10−6 | 100%,102 high103,104 | |
| Ni | 2 × 10−4–8 × 10−4 | 99%,105 high,106,107 >60%108 | |
| Sb | 0.006–0.01 | >90%,c109 80%c110 | |
| Sn | 0.03–0.06 | ||
| Ti | 0.5–2 | 100%,111 99%,112 high113 | |
| Zr | 0.2–4 | 93%114 | |
| Anthropogenic flows dominate element mobilization | Al | 0.005–0.01 | High113,115,116 |
| Co | 0.003–0.005 | 95%d117 | |
| Mg | 1 × 10−4–2 × 10−4 | 100%,e118 95%f119 | |
| Mn | 8 × 10−6–1 × 10−5 | 99%,g120 91%,121 62%,122 60%123 | |
| Si | 3–25 | 100%124 | |
| Zn | 0.04–0.2 | 87–98%,125 high94 | |
| C: | |||
| CNTs | <10%126 | ||
| Graphene | 8%h127 | ||
| Fullerenes | 15–33%128 | ||
| Diamonds | 15%129 | ||
Several other interesting features emerge from the anthropogenic lifecycle evaluation. The commodity metals (Fe, Cu, and Al) tend to be extracted with great efficiency from their ores, with only 17, 18, and 21% going to tailings and slag, respectively. Silver, cerium, and nickel have higher losses to tailings and slag (27, 28, and 29%, respectively). Silver is not a “carrier metal” with a primary ore source, and it is usually extracted as a co-product during lead, copper, and tin processing.130 Cerium is a rare earth element (REE) and thus occurs in highly mixed ore deposits with other REEs.70 (Refer to the ESI† for a detailed discussion on the high losses to tailings and slag calculated for nickel).
Cobalt's extremely low extraction efficiency stands out among the commercially and technologically important elements investigated here (Fig. 4); it has estimated losses of 63% to tailings and slag. First, cobalt is mined as a byproduct of other primary minerals or metals (e.g., nickel laterite ore) and can be recovered from previously stock piled materials. Therefore, cobalt recovery rates are highly variable and depend on the extractive metallurgical techniques used for both the primary metal as well as cobalt and associated variables (e.g., type of ore, energy availability, and market demands).72 Second, the low extraction efficiencies also reflect the rudimentary extraction technologies used to produce ore and concentrates, a dominant fraction of which is mined in the Democratic Republic of Congo (58.2%; with Russia, Australia, Canada, and Cuba supplying small fractions of 5.1, 4.5, 3.9, and 3.8%, respectively in estimated 2017 values).131 Recent studies have highlighted the sensitivity of cobalt to supply chain limitation,132 with a particular emphasis on the growing need for cobalt in LiNiCo Li-ion batteries used in advanced electronics (including laptops, cellphones, and automobiles). Indeed, the price of cobalt more than quadrupled between 2016 and 2018 (from just over a low of $20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to just under a high of $100
000 to just under a high of $100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per metric ton, falling to approximately $30
000 per metric ton, falling to approximately $30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 by early 2019).133 This economic driver will continue to enhance recycling rates of cobalt, which are already at 8%.
000 by early 2019).133 This economic driver will continue to enhance recycling rates of cobalt, which are already at 8%.
Aluminum, nickel, silver, and iron are all well recycled (41, 42, 45, and 61%, respectively), which reflects not only the value and scale of the established industries, but also the relative simplicity of their commercial products.134 In stark contrast, copper is recycled at lower rates (only 27%). While the bulk of stockpiled copper is present in building materials, with long useful lifetimes and many practical limitations to recycle, the dominant usage is shifting to a pervasive presence in electronics and electronic infrastructure.74 Thus, low recycle rates may be a consequence of the complexity of the commercial sources of secondary copper metals: it is not economically viable to extract an element that is integrated within a diverse mixture of other components. Dahmus and Gutowski134 were first to highlight this with the development of a material mixing metric (“H (bits)”), noting that laptops, desktops, cellphones, and other advanced electronics have prohibitive material complexities with relatively low economic value of the produced recycled materials. Thus, the economic incentive to disassemble these devices and recover bulk elements is not competitive. Indeed, even with manual or machine-assisted disassembly, the technologies to separate the metals from their internal components can be limiting135 (especially for elements like the rare earth and specialty metals136). This technological challenge of metal separation and recovery is one reason why metals like cerium (a rare earth element) are functionally unrecycled. REE recycling potential remains low136 for applications in which REEs are used in small quantities and complex devices.70 However, larger volume applications (e.g., automobile catalysts, magnets, and polishing slurries) are potentially recyclable (e.g., Nd, Dy, and Ce).70,136,137 Here, we note that REE recycling technology is an active area of research in private, government,138,139 academic140–143 and industry sectors,144 and the US Department of Energy145 is making substantial investments in recovery technologies, not only to overcome metal criticality, but also to improve national security as related to renewable energy and advanced electronic technologies.
Current nano-enabled technologies are largely immune to these metal criticality concerns (except for nanoceria, nanocobalt oxides, and nanoantimony); the relatively small nanomarkets are functionally buffered by the proportionately large size of mined materials (e.g., Fig. 4). Of course, this analysis has a few potential oversights: (1) rapid market growth might eventually lead to some material limitation, but presumably at a predictable rate, and (2) the nanomaterial market size captures only a fraction of the material that needs to be mined in order to make those products due to inefficiencies in nanofabrication (e.g., as in Table 3). In order to evaluate the market trends in nanomaterial production, we considered annual production volume growth rates.
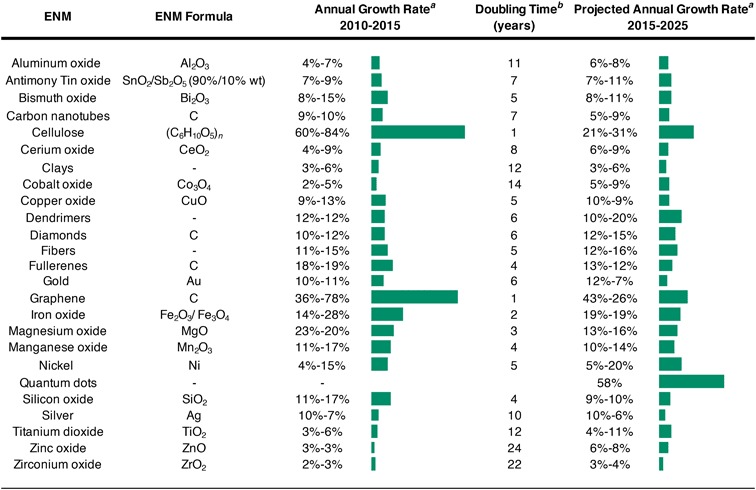
Despite the minor influence of ENMs on anthropogenic element cycling, we find non-trivial exponential production growth rates. First, from 2010–2015, the majority of nanomaterials experienced growth rates below 2–20% (Table 4). The fastest growing markets at the time were for iron oxide (14–28% annual growth rate (AGR)), magnesium oxide (23–20% AGR), graphene (36–78% AGR), and cellulose (60–84% AGR). Notably, the market for graphene and cellulose doubled in volume approximately every year. For the integrated period of 2015–2025, the largest market expansions are predicted for dendrimers and zinc oxides, each of which could double their modest growth rates (to 10–20% and 6–8% AGR, respectively), and QDs, which are anticipated to have 58% AGR over 2015–2025. Beyond these standouts, the rates of market growth for the majority of nanomaterials are expected to stagnate or shrink for the majority of nanomaterials. Considering these growth rates, 35–180 years of sustained market expansion (an unlikely event) would be required before nanomaterial markets could dominate anthropogenic element cycles, even without accounting for growth in bulk element mobilizations. This illustrates that nanomaterials are not anticipated to dominant anthropogenic mobilizations in the foreseeable future, suggesting nanomaterials have a minor influence on global-scale processes but may be important in local disturbances or exposure events.
| a Exponential annual growth rates are presented as a percentage calculated using eqn (1). b Doubling times are calculated using annual growth rates (2010–2015) from high production volume estimates (Future Markets Inc., 2016). |
|---|
|
Second, accounting for the influence of synthetic inefficiencies on required mining to support a given market, we note that reported yields for metal and metalloid nanomaterials are high (usually higher than 60% but often greater than 90% metal conversion as reported in academic journals (Table 3)), suggesting that nanomarket sizes are not substantially underestimating the amount of material required to produce them. The carbon-based nanomaterials, while typically produced from petrochemically-derived gases146 rather than mined minerals, have dramatically lower synthetic efficiencies: 8, less than 10, 15, and 15–33% carbon conversion yield for graphene, CNTs, nanodiamonds, and fullerenes (Table 3). Several synthetic strategies (such as lowering the gas-to-metal catalyst ratio) allow for improvement of these yields,126,147,148 and these deserve focused research.
While not useful for a calculation of mined primary materials for nanomaterial fabrication, another metric to gauge industrial process efficiency is the waste-to-product ratios (E-factor analysis). E-Factor analysis accounts for all materials used in the manufacturing process, including washes or purification steps, and these are reported on a mass-ratio basis. Typically, E-factors for large-scale industries range from 0.1 for oil refining, less than 1 to 5 for bulk chemicals, 5–50 for fine chemicals, and 25 to over 100 for pharmaceuticals,149,150 whereas nanomaterial production and purification has E-factors in the range of 100–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000151 for metal, metalloid, and carbon-based nanomaterials; i.e., these are among the most wasteful industrial syntheses of our time. Here, we note that E-factors150,152,153 tend to anticorrelate with the age and throughput of an industry, where processes become more refined and efficient with time and at scale (i.e., all factors related to TRL), either due to discovery or utilization of waste streams for other marketable purposes. However, recent development trends show that technologies tend to be displaced prior to this optimization, as noted by energy metrics highlighted in Gutowski et al.154 For nanomaterials, even though the industries have grown over the last decade, it is unclear that any dramatic improvements in E-factor (or yields for the carbon-based nanomaterials) have occurred. Thus, nanotechnology environmental health and safety researchers devoted to reducing the environmental impact of ENMs should focus efforts on developing more benign materials syntheses.
000151 for metal, metalloid, and carbon-based nanomaterials; i.e., these are among the most wasteful industrial syntheses of our time. Here, we note that E-factors150,152,153 tend to anticorrelate with the age and throughput of an industry, where processes become more refined and efficient with time and at scale (i.e., all factors related to TRL), either due to discovery or utilization of waste streams for other marketable purposes. However, recent development trends show that technologies tend to be displaced prior to this optimization, as noted by energy metrics highlighted in Gutowski et al.154 For nanomaterials, even though the industries have grown over the last decade, it is unclear that any dramatic improvements in E-factor (or yields for the carbon-based nanomaterials) have occurred. Thus, nanotechnology environmental health and safety researchers devoted to reducing the environmental impact of ENMs should focus efforts on developing more benign materials syntheses.
Conclusions and implications
This article is (1) first to consider ENMs within the framework of anthropogenic element cycling of bulk materials at the global level, (2) provides an update to relevant and emergent nanomaterial applications and market sizes, (3) synthesizes an accounting of anthropogenic cycling for nano-relevant material sources, and (4) reviews synthetic nanomaterial fabrication efficiencies that have EHS and materials extraction implications.From a global perspective, nanomaterial markets make up relatively small proportions of in-use materials. This implies that engineered nanomaterials, when distributed in the environment or over the globe, would have a negligible effect compared to their natural analogs. Thus, Garrels'11 concept of elemental cycle disturbances leading to toxic outcomes is most relevant for local events, such as occupational exposure or point-source releases of ENMs. In any case, analytical tools able to discern engineered from natural nanoparticles remain limited,155–157 where substantial challenge is presented by the disparity in concentration between ENMs and NNMs/INMs, the latter of which exist at higher abundances. Here, it's worth noting that the toxicity of some ENMs is dramatically distinct from their natural analogs (e.g., CNTs, where morphology presents unique respiratory toxicity beyond that of spherical or agglomerated black carbon soots), and so there is strong motivation to be able to distinguish between the two forms of highly reduced carbon. The same urgency may not exist for metal and metalloid nanomaterials, which may lack the feature of nano-specific toxicity or environmental impact (e.g., Ag) or are identifiable as industrially sourced (e.g., QDs). For example, QDs will have toxicity related to their metal constituents, but also contain elemental ratios that are sufficiently unique from natural minerals to potentially identify them in the environment (until the atoms dissociate). Considering the implicit toxicity of some QDs and the rapid anticipated market growth, research to understand the environmental fate of these materials is justified. Here, we note that effort has been expended to facilitate QD recovery in response to REACH, but this will not be the case for medical nanotechnologies, which rely on both exposure and subsequent release of the materials into waste treatment streams (e.g., see prior contributions of the Westerhoff group158–160 for metallic and metal oxide nanoparticles released to waste treatment streams).
Taken together, our work suggests that nanomaterial markets and applications continue to evolve, and there is great opportunity to incorporate green engineering design principles into ENM-enabled technologies. Specifically, molecular design strategies to eliminate the inherent risk associated with ENMs and their fabrication approaches must continue. In particular, we emphasize that many unique applications of nanomaterials continue to emerge, and those contained in a large variety of applications may have the most diverse exposure routes (i.e., they represent substantial EHS research challenges). Here, we stress the need for focused EHS research and enhanced design guided by green engineering135 to (1) discern ENMs with risks unique to the nanoform and (2) improve molecular design to eliminate the inherent hazard. Finally, we underscore that when hazard is unique to the nanoform, a mass-based comparison with bulk materials is not the appropriate metric to contextualize impact.
Probing the nanomaterial space en masse, one can prioritize EHS research by element type and suggest the following: (1) for metals, current and future markets are expected to remain minor relative to other anthropogenic uses. QDs provide an exception with rapid anticipated growth and few other technologies that rely on those most common QD elements (Cd, Se, and Te). (2) For metal oxides, the nanomaterial markets are substantial and represent the largest proportion of any ENM in a material's use phase (e.g., 3–25% of the total Si market). They also have some of the highest anticipated growth rates in the coming years. Thus, metal oxide ENMs may have the greatest environmental abundance on a mass basis. (3) For carbonaceous nanomaterials, especially nanocellulose and graphene, there is strong anticipated growth of the industries with many diverse applications (i.e., and corresponding exposure routes). Further, the synthetic efficiencies for some of these processes tend to be extremely low. Therefore, EHS research should continue to work to improve industrial processing for carbonaceous ENMs in order to mitigate the geochemical impact of these emergent technologies.
Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgements
We thank the National Science Foundation Graduate Research Fellowship Program for support of N. Z. J. and Drs. Thomas E. Graedel, Barbara K. Reck, Michael Hochella and Christine Ogilvie Hendren for valuable conversations regarding this work. We thank anonymous reviewers for suggestions to improve the manuscript. Graphical abstract created by N. Z. J. and inspired by Luca Ciacci et al.'s Lost by Design.136References
- ECHA, Eur. Chem. Agency, Reach, 2016, vol. 2.1, p. 496 Search PubMed.
- M. Wiesner, K. Jones, G. Lowry, R. Di Giulio and M. Hochella, Award #0830093 Center for Environmental Implications of Nanotechnology, NSF Award Search Database, Accessed 09/26/2018.
- M. Wiesner, K. Jones, G. Lowry, R. Di Giulio and M. Hochella, Award #1266252 Center for the Environmental Implications of NanoTechnology (CEINT), NSF Award Search Database, Accessed 09/26/2018.
- A. Nel, H. Godwin, R. Nisbet, Y. Cohen and A. Keller, Award #0830117, CEIN: Predictive Toxicology Assessment and Safe Implementation of Nanotechnology in the Environment, NSF Award Search Database, Accessed 09/26/2018.
- A. Nel, H. Godwin, Y. Cohen, A. Keller and P. Holden, Award #1266377 University of California Center for Environmental Implications of Nanotechnology (UC-CEIN) NSF Award Search Database, Accessed 09/26/2018.
- Award # RD ¬ 83558001-¬0 Lifecycle of Nanomaterials (LC Nano), EPA Grant Award Database, Accessed 09/26/2019.
- M. M. Falinski, D. L. Plata, S. S. Chopra, T. L. Theis, L. M. Gilbertson and J. B. Zimmerman, Nat. Nanotechnol., 2018, 13, 708–714 CrossRef CAS PubMed.
- National Science and Technology Council, Committee on Technology (COT) and Subcommittee on Nanoscale Science Engineering and Technology (NSET), The National Nanotechnology Initiative Supplement to the President’s Budget For Fiscal Year 2018, 2017 Search PubMed.
- National Science and Technology Council, Committee on Technology (COT) and Subcommittee on Nanoscale Science Engineering and Technology (NSET), The National Nanotechnology Initiative Supplement to the President's Budget For Fiscal Year 2019, 2018 Search PubMed.
- H. Wigger, W. Wohlleben and B. Nowack, Environ. Sci.: Nano, 2018, 5, 1372–1385 RSC.
- R. M. Garrels, F. T. Mackenzie and C. Hunt, Chemical Cycles and the Global Environment: Assessing Human Influences, W. Kaufman, Inc., Los Altos, California, 1975 Search PubMed.
- U. S. Environmental Protection Agency (EPA), 2012 Edition of the Drinking Water Standards and Health Advisories, Washington, DC, 2012 Search PubMed.
- World Health Organization (WHO), Guidelines for Drinking-Water Quality, Fourth Edition, World Health Organization, 4th edn, 2011 Search PubMed.
- G. F. Nordberg, B. A. Fowler and M. Nordberg, Handbook on the Toxicology of Metals: Fourth Edition, Elsevier B.V., 4th edn, 2014, vol. 1–2 Search PubMed.
- A. Chopra and C. Lineweaver, Proc. 8th Aust. Sp. Sci. Conf., 2008, pp. 49–55 Search PubMed.
- A. Banin and J. Navrot, Science, 1975, 189, 550–551 CrossRef CAS PubMed.
- U.S. Department of Health and Human Services, Toxicology and Carcinogenesis Studies of Sodium Dichromate Dihydrate, 2008 Search PubMed.
- M. Edwards, S. Triantafyllidou and D. Best, Environ. Sci. Technol., 2009, 43, 1618–1623 CrossRef CAS PubMed.
- K. J. Pieper, M. Tang and M. A. Edwards, Environ. Sci. Technol., 2017, 51, 2007–2014 CrossRef CAS PubMed.
- R. Nickson, J. McArthur, W. Burgess, K. Matin Ahmed, P. Ravenscroft and M. Rahman, Nature, 1998, 395, 338 CrossRef CAS PubMed.
- T. R. Chowdhury, G. K. Basu, B. K. Mandal, B. K. Biswas, G. Samanta, U. K. Chowdhury, C. R. Chanda, D. Lodh, S. L. Roy, K. C. Saha, S. Roy, S. Kabir, Q. Quamruzzaman and D. Chakraborti, Nature, 1999, 401, 545–546 CrossRef CAS PubMed.
- F. S. Islam, A. G. Gault, C. Boothman, D. A. Polya, J. M. Chamok, D. Chatterjee and J. R. Lloyd, Nature, 2004, 430, 68–71 CrossRef CAS PubMed.
- C. H. Lamborg, C. R. Hammerschmidt, K. L. Bowman, G. J. Swarr, K. M. Munson, D. C. Ohnemus, P. J. Lam, L.-E. Heimbürger, M. J. A. Rijkenberg and M. A. Saito, Nature, 2014, 512, 65–68 CrossRef CAS PubMed.
- H. J. Segall and J. M. Wood, Nature, 1974, 248, 456–458 CrossRef CAS PubMed.
- C. Y. Usenko, S. L. Harper and R. L. Tanguay, Carbon, 2007, 45, 1891–1898 CrossRef CAS PubMed.
- L. Truong, T. Zaikova, E. K. Richman, J. E. Hutchison and R. L. Tanguay, Nanotoxicology, 2012, 6, 691–699 CrossRef CAS PubMed.
- M. C. Arnold, A. R. Badireddy, M. R. Wiesner, R. T. Di Giulio and J. N. Meyer, Arch. Environ. Contam. Toxicol., 2013, 65, 224–233 CrossRef CAS PubMed.
- H. Zhang, Z. Ji, T. Xia, H. Meng, C. Low-Kam, R. Liu, S. Pokhrel, S. Lin, X. Wang, Y. P. Liao, M. Wang, L. Li, R. Rallo, R. Damoiseaux, D. Telesca, L. Mädler, Y. Cohen, J. I. Zink and A. E. Nel, ACS Nano, 2012, 6, 4349–4368 CrossRef CAS PubMed.
- M. A. Maurer-Jones, I. L. Gunsolus, C. J. Murphy and C. L. Haynes, Anal. Chem., 2013, 85, 3036–3049 CrossRef CAS PubMed.
- K. H. Liao, Y. S. Lin, C. W. MacOsko and C. L. Haynes, ACS Appl. Mater. Interfaces, 2011, 3, 2607–2615 CrossRef CAS PubMed.
- L. M. Pasquini, S. M. Hashmi, T. J. Sommer, M. Elimelech and J. B. Zimmerman, Environ. Sci. Technol., 2012, 46, 6297–6305 CrossRef CAS PubMed.
- T. Xia, M. Kovochich, J. Brant, M. Hotze, J. Sempf, T. Oberley, C. Sioutas, J. I. Yeh, M. R. Wiesner and A. E. Nel, Nano Lett., 2006, 6, 1794–1807 CrossRef CAS PubMed.
- N. Gou, A. Onnis-Hayden and A. Z. Gu, Environ. Sci. Technol., 2010, 44, 5964–5970 CrossRef CAS PubMed.
- J. Lan, N. Gou, C. Gao, M. He and A. Z. Gu, Environ. Sci. Technol., 2014, 48, 12937–12945 CrossRef CAS PubMed.
- A. E. Nel, L. Mädler, D. Velegol, T. Xia, E. M. V. Hoek, P. Somasundaran, F. Klaessig, V. Castranova and M. Thompson, Nat. Mater., 2009, 8, 543–557 CrossRef CAS PubMed.
- A. Nel, T. Xia, H. Meng, X. Wang, S. Lin, Z. Ji and H. Zhang, Acc. Chem. Res., 2013, 46, 607–621 CrossRef CAS PubMed.
- A. A. Dayem, M. K. Hossain, S. Bin Lee, K. Kim, S. K. Saha, G. M. Yang, H. Y. Choi and S. G. Cho, Int. J. Mol. Sci., 2017, 18, 1–21 Search PubMed.
- C. A. Poland, R. Duffin, I. Kinloch, A. Maynard, W. A. H. Wallace, A. Seaton, V. Stone, S. Brown, W. MacNee and K. Donaldson, Nat. Nanotechnol., 2008, 3, 423–428 CrossRef CAS PubMed.
- G. Oberdörster, V. Castranova, B. Asgharian and P. Sayre, J. Toxicol. Environ. Health, Part B, 2015, 18, 121–212 Search PubMed.
- M. F. Hochella, D. W. Mogk, J. Ranville, I. C. Allen, G. W. Luther, L. C. Marr, B. P. McGrail, M. Murayama, N. P. Qafoku, K. M. Rosso, N. Sahai, P. A. Schroeder, P. Vikesland, P. Westerhoff and Y. Yang, Science, 2019, 363(6434), eaau8299 CrossRef PubMed.
- Z. M. Xiu, Q. B. Zhang, H. L. Puppala, V. L. Colvin and P. J. J. Alvarez, Nano Lett., 2012, 12, 4271–4275 CrossRef CAS PubMed.
- T. C. King-Heiden, P. N. Wiecinski, A. N. Mangha, K. M. Metz, D. Nesbit, J. A. Pedersen, R. J. Hamers, W. Heideman and R. E. Peterson, Environ. Sci. Technol., 2009, 43, 1605–1611 CrossRef CAS PubMed.
- X. Yang, A. P. Gondikas, S. M. Marinakos, M. Auffan, J. Liu, H. Hsu-Kim and J. N. Meyer, Environ. Sci. Technol., 2012, 46, 1119–1127 CrossRef CAS PubMed.
- B. Song, J. Liu, X. Feng, L. Wei and L. Shao, Nanoscale Res. Lett., 2015, 10, 342 CrossRef PubMed.
- Z. Lin, N. A. Monteiro-Riviere and J. E. Riviere, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2015, 7, 189–217 CAS.
- F. Piccinno, F. Gottschalk, S. Seeger and B. Nowack, J. Nanopart. Res., 2012, 14, 1109 CrossRef.
- B. Giese, F. Klaessig, B. Park, R. Kaegi, M. Steinfeldt, H. Wigger, A. Von Gleich and F. Gottschalk, Sci. Rep., 2018, 8, 1–18 CrossRef CAS PubMed.
- F. Gottschalk, T. Sun and B. Nowack, Environ. Pollut., 2013, 181, 287–300 CrossRef CAS PubMed.
- T. Y. Sun, G. Conroy, E. Donner, K. Hungerbühler, E. Lombi and B. Nowack, Environ. Sci.: Nano, 2015, 2, 340–351 RSC.
- A. A. Keller and A. Lazareva, Environ. Sci. Technol. Lett., 2014, 1, 65–70 CrossRef CAS.
- R. J. Klee and T. E. Graedel, Annu. Rev. Environ. Resour., 2004, 29, 69–107 CrossRef.
- I. S. Sen and B. Peucker-Ehrenbrink, Environ. Sci. Technol., 2012, 46, 8601–8609 CrossRef CAS PubMed.
- S. Bringezu and Y. Moriguchi, A Handbook of Industrial Ecology, 2002 Search PubMed.
- W.-Q. Chen and T. E. Graedel, Environ. Sci. Technol., 2012, 46, 8574–8586 CrossRef CAS PubMed.
- M. J. Eckelman and T. E. Graedel, Environ. Sci. Technol., 2007, 41, 6283–6289 CrossRef CAS PubMed.
- A. Caballero-Guzman and B. Nowack, Environ. Pollut., 2016, 213, 502–517 CrossRef CAS PubMed.
- Future Markets Inc., The Global Market for Nanotechnology and Nanomaterials, 2016 Search PubMed.
- U.S. Department of Energy, Standard Review Plan (SRP) Technology Readiness Assessment Report, 2010 Search PubMed.
- Nanotechnology Knowledge Infrastructure (NKI) Signature Initiative, Data Readiness Levels, Nanotechnology Signature Initiative Nanotechnology Knowledge Infrastructure (NKI): Enabling National Leadership in Sustainable Design, 2013 Search PubMed.
- E. L. Bray, 2016 USGS Mineral Commodity Summaries, Magnesium Compounds, 2016 Search PubMed.
- E. L. Bray, 2016 USGS Mineral Commodity Summaries, Magnesium Metal, 2016 Search PubMed.
- USGS, USGS Magnesium Statistics and Information, https://minerals.usgs.gov/minerals/pubs/commodity/magnesium/index.html, (accessed 12 March 2019) Search PubMed.
- U.S. Geological Survey, 2014 USGS Minerals Yearbook, Silicon, Vol. I. Metals and Minerals, 2016 Search PubMed.
- E. K. Schnebele, 2016 USGS Mineral Commodity Summaries, Silicon, 2016 Search PubMed.
- B. K. Reck and T. E. Graedel, Science, 2012, 337, 690–695 CrossRef CAS PubMed.
- J. Johnson, J. Jirikowic, M. Bertram, D. Van Beers, R. B. Gordon, K. Henderson, R. J. Klee, T. Lanzano, R. Lifset, L. Oetjen and T. E. Graedel, Environ. Sci. Technol., 2005, 39, 4655–4665 CrossRef CAS PubMed.
- U.S. Geological Survey, 2014 USGS Minerals Yearbook, Silver, Vol. I. Metals and Minerals, 2016 Search PubMed.
- G. Liu, C. E. Bangs and D. B. Müller, Nat. Clim. Change, 2012, 3, 338–342 CrossRef.
- U.S. Geological Survey, 2015 USGS Minerals Yearbook, Aluminum, Vol. I. Metals and Minerals, 2016 Search PubMed.
- X. Du and T. E. Graedel, Sci. Rep., 2011, 1, 145 CrossRef PubMed.
- U.S. Geological Survey, 2014 USGS Minerals Yearbook, Rare Earths, Vol. I. Metals and Minerals, 2016 Search PubMed.
- E. M. Harper, G. Kavlak and T. E. Graedel, Environ. Sci. Technol., 2012, 46, 1079–1086 CrossRef CAS PubMed.
- U.S. Geological Survey, 2014 USGS Minerals Yearbook, Cobalt, Vol. I. Metals and Minerals, 2016 Search PubMed.
- S. Glöser, M. Soulier and L. A. Tercero Espinoza, Environ. Sci. Technol., 2013, 47, 6564–6572 CrossRef PubMed.
- U.S. Geological Survey, 2014 USGS Minerals Yearbook, Copper, Vol. I. Metals and Minerals, 2016 Search PubMed.
- International Copper Study Group, The World Copper Factbook, 2015 Search PubMed.
- T. Wang, D. B. Muller and T. E. Graedel, Environ. Sci. Technol., 2007, 41, 5120–5129 CrossRef CAS PubMed.
- U.S. Geological Survey, 2014 USGS Minerals Yearbook, Iron Ore, Vol. I. Metals and Minerals, 2017 Search PubMed.
- P. H. Kuck, 2016 USGS Mineral Commodity Summaries, Nickel, 2016 Search PubMed.
- International Nickel Study Group (INSG), World Nickel Statistics 2015, 2015 Search PubMed.
- G. Meylan and B. K. Reck, Resour., Conserv. Recycl., 2015, 1–10 Search PubMed.
- U.S. Geological Survey, 2014 USGS Minerals Yearbook, Zinc, Vol. I. Metals and Minerals, 2016 Search PubMed.
- D. I. Bleiwas and J. Gambogi, USGS Preliminary Estimates of the Quantities of Rare-Earth Elements Contained in Selected Products and in Imports of Semimanufactured Products to the United States, 2010, 2013 Search PubMed.
- L. Z. H. I. Li and X. Yang, 1st Eur. Rare Earth Resour. Conf., 2014, pp. 26–36 Search PubMed.
- B. K. Reck and V. S. Rotter, J. Ind. Ecol., 2012, 16, 518–528 CrossRef CAS.
- A. A. Keller, S. McFerran, A. Lazareva and S. Suh, J. Nanopart. Res., 2013, 15, 1692 CrossRef.
- R. P. Schwarzenbach, P. M. Gschwend and D. M. Imboden, Environmental Organic Chemistry, John Wiley & Sons, Inc., 3rd edn, 2017 Search PubMed.
- Industry Experts, Polytetrafluoroethylene (PTFE) – A Global Market Overview, 2016 Search PubMed.
- S. Coe-Sullivan, W. Liu, P. Allen and J. S. Steckel, ECS J. Solid State Sci. Technol., 2012, 2, R3026–R3030 CrossRef.
- E. Oh, I. L. Medintz, R. Liu, M. Bilal, Y. Cohen, K. B. Gemill and A. Nel, Nat. Nanotechnol., 2016, 11, 479–486 CrossRef CAS PubMed.
- J. Kiventera, L. Golek, J. Yliniemi, V. Ferreira, J. Deja and M. Illikainen, Int. J. Miner. Process., 2016, 149, 104–110 CrossRef CAS.
- M. Fall, M. Benzaazoua and S. Ouellet, Proc. eighth Int. Symp. Min. with backfill (Minefill 2004), 2004, pp. 193–202 Search PubMed.
- L. Pourzahedi and M. J. Eckelman, Environ. Sci.: Nano, 2015, 2, 361–369 RSC.
- J. A. Dahl, B. L. S. Maddux and J. E. Hutchison, Chem. Rev., 2007, 107, 2228–2269 CrossRef CAS PubMed.
- Y. Sun, Chem. Soc. Rev., 2013, 42, 2497–2511 RSC.
- P. Zhao, N. Li and D. Astruc, Coord. Chem. Rev., 2013, 257, 638–665 CrossRef CAS.
- W. Li, Mater. Chem. Phys., 2006, 99, 174–180 CrossRef CAS.
- C. Hu, Z. Zhang, H. Liu, P. Gao and Z. L. Wang, Nanotechnology, 2006, 17, 5983–5987 CrossRef CAS.
- Z.-R. Tang, Y. Zhang and Y.-J. Xu, RSC Adv., 2011, 1, 1772 RSC.
- Y. Lee, J. Choi, K. J. Lee, N. E. Stott and D. Kim, Nanotechnology, 2008, 19, 415604 CrossRef PubMed.
- Z. s. Hong, Y. Cao and J. f. Deng, Mater. Lett., 2002, 52, 34–38 CrossRef CAS.
- Z. Cui, L. Jiang, W. Song and Y. Guo, Chem. Mater., 2009, 21, 1162–1166 CrossRef CAS.
- S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064–2110 CrossRef CAS PubMed.
- W. Wu, Q. He and C. Jiang, Nanoscale Res. Lett., 2008, 3, 397–415 CrossRef CAS PubMed.
- J. W. Seo, H. S. Nam, A. S. Im, K. M. Kim and J. J. Lee, US Pat., US 2010/0031775, 2010 Search PubMed.
- N. Sahiner, H. Ozay, O. Ozay and N. Aktas, Appl. Catal., A, 2010, 385, 201–207 CrossRef CAS.
- X. Hu and J. C. Yu, Chem. Mater., 2008, 20, 6743–6749 CrossRef CAS.
- E. J. Roberts, S. E. Habas, L. Wang, D. A. Ruddy, E. A. White, F. G. Baddour, M. B. Griffin, J. A. Schaidle, N. Malmstadt and R. L. Brutchey, ACS Sustainable Chem. Eng., 2017, 5, 632–639 CrossRef CAS.
- M. Zheng and B. Wang, Trans. Nonferrous Met. Soc. China, 2009, 19, 404–409 CrossRef CAS.
- Y. Li, J. Wang, B. Feng, K. Duan and J. Weng, J. Alloys Compd., 2015, 634, 37–42 CrossRef CAS.
- T. Sugimoto, X. Zhou and A. Muramatsu, J. Colloid Interface Sci., 2003, 259, 43–52 CrossRef CAS PubMed.
- A. Y. Vakayil, S. M. Bashir and F. M. S. Trabzuni, US Pat., US 8454932 B2, 2013 Search PubMed.
- F. Kamil, J. Nanosci. Technol., 2016, 2, 37–39 Search PubMed.
- M. Mizuno, Y. Sasaki, S. Lee and H. Katakura, Langmuir, 2006, 22, 7137–7140 CrossRef CAS PubMed.
- A. A. Efimov, A. A. Lizunova, I. A. Volkov, D. A. Mylnikov, P. V. Arsenov and V. V. Ivanov, J. Phys.: Conf. Ser., 2016, 741, 1 CrossRef.
- A. K. Patra, A. Dutta and A. Bhaumik, J. Hazard. Mater., 2012, 201–202, 170–177 CrossRef CAS PubMed.
- Y. Hou, H. Kondoh, M. Shimojo, T. Kogure and T. Ohta, J. Phys. Chem. B, 2005, 109, 19094–19098 CrossRef CAS PubMed.
- S. M. Maliyekkal, Anshup, K. R. Antony and T. Pradeep, Sci. Total Environ., 2010, 408, 2273–2282 CrossRef CAS PubMed.
- S. Makhluf, R. Dror, Y. Nitzan, Y. Abramovich, R. Jelinek and A. Gedanken, Adv. Funct. Mater., 2005, 15, 1708–1715 CrossRef CAS.
- D. Jing, D. Chen, G. Fan, Q. Zhang, J. Xu, S. Gou, H. Li and F. Nie, Cryst. Growth Des., 2016, 16, 6849–6857 CrossRef CAS.
- L. Lan, G. Gu, Q. Li, H. Zhang, K. Xu, B. Liu and B. Liu, RSC Adv., 2015, 5, 25250–25257 RSC.
- A. L. Willis, Z. Chen, J. He, Y. Zhu, N. J. Turro and S. O'Brien, J. Nanomater., 2007, 2007, 14858 CrossRef.
- M. Salavati-Niasari, F. Davar and M. Mazaheri, Polyhedron, 2008, 27, 3467–3471 CrossRef CAS.
- C. Takai, T. Ishino, M. Fuji and T. Shirai, Colloids Surf., A, 2014, 446, 46–49 CrossRef CAS.
- S. López-Cuenca, L. A. Pérez Carrillo, M. Rabelero Velasco, R. Díaz De Len, H. Saade, R. G. López, E. Mendizábal and J. E. Puig, J. Nanomater., 2011, 2011, 431382 Search PubMed.
- L. M. Gilbertson, J. B. Zimmerman, D. L. Plata, J. E. Hutchison and P. T. Anastas, Chem. Soc. Rev., 2015, 44, 5758–5777 RSC.
- I. Janowska, D. Bégin, O. Ersen, M. S. Moldovan, K. Chizari, M. J. Ledoux and C. Pham-Huu, Phys. E, 2012, 44, 1009–1011 CrossRef CAS.
- M. M. Kasumov, Int. J. Appl. Math. Comput. Sci., 2015, 1, 166–173 Search PubMed.
- J.-P. Boudou, P. A. Curmi, F. Jelezko, J. Wrachtrup, P. Aubert, M. Sennour, G. Balasubramanian, R. Reuter, A. Thorel and E. Gaffet, Nanotechnology, 2009, 20, 359801–359801 CrossRef.
- T. E. Graedel and B. R. Allenby, Industrial Ecology and Sustainable Engineering, Prentice Hall, 2010 Search PubMed.
- K. B. Shedd, U.S. Geological Survey, Mineral Commodity Summaries, Cobalt, 2018 Search PubMed.
- E. A. Olivetti, G. Ceder, G. G. Gaustad and X. Fu, Joule, 2017, 1, 229–243 CrossRef.
- LME Cobalt, Historical Price Graph, https://www.lme.com/en-GB/Metals/Minor-metals/Cobalt#tabIndex=2, (accessed 20 February 2019) Search PubMed.
- J. B. Dahmus and T. G. Gutowski, Environ. Sci. Technol., 2007, 41, 7543–7550 CrossRef CAS PubMed.
- M. P. O'Connor, J. B. Zimmerman, P. T. Anastas and D. L. Plata, ACS Sustainable Chem. Eng., 2016, 4, 5879–5888 CrossRef.
- L. Ciacci, B. K. Reck, N. T. Nassar and T. E. Graedel, Environ. Sci. Technol., 2015, 49, 9443–9451 CrossRef CAS PubMed.
- Umicore, Cerium Applications Search PubMed.
- REECover (European Commission), Recovery of Rare Earth Elements from magnetic waste in the WEEE recycling industry and tailings from the iron ore industry, 2016 Search PubMed.
- European Commission/European Union's Horizon 2020 Research and Innovation Program, 2019, pp. 4–7 Search PubMed.
- K. Binnemans, P. T. Jones, T. Müller and L. Yurramendi, J. Sustain. Met., 2018, 4, 126–146 CrossRef.
- M. Reimer, H. Schenk-Mathes, M. Hoffmann and T. Elwert, Metals, 2018, 8, 867 CrossRef.
- C. Borra, T. Vlugt, Y. Yang and S. Offerman, Metals, 2018, 8, 801 CrossRef.
- M. P. O'Connor, R. M. Coulthard and D. L. Plata, Environ. Sci.: Water Res. Technol., 2018, 4, 58–66 RSC.
- Umicore and Rhodia, Press Release CP-2011-18-R, 2011, pp. 31–32 Search PubMed.
- US DOE, National Energy Technology Laboratory Rare Earth Elements Program, 2018 Search PubMed.
- D. L. Plata, P. M. Gschwend and C. M. Reddy, Nanotechnology, 2008, 19, 18 CrossRef PubMed.
- R. Rao, C. L. Pint, A. E. Islam, R. S. Weatherup, S. Hofmann, E. R. Meshot, F. Wu, C. Zhou, N. Dee, P. B. Amama, J. Carpena-Nuñez, W. Shi, D. L. Plata, E. S. Penev, B. I. Yakobson, P. B. Balbuena, C. Bichara, D. N. Futaba, S. Noda, H. Shin, K. S. Kim, B. Simard, F. Mirri, M. Pasquali, F. Fornasiero, E. I. Kauppinen, M. Arnold, B. A. Cola, P. Nikolaev, S. Arepalli, H. M. Cheng, D. N. Zakharov, E. A. Stach, J. Zhang, F. Wei, M. Terrones, D. B. Geohegan, B. Maruyama, S. Maruyama, Y. Li, W. W. Adams and A. J. Hart, ACS Nano, 2018, 12, 11756–11784 CrossRef CAS PubMed.
- T. Osawa, K. Hasegawa, S. Noda, Z. Chen and D. Y. Kim, Carbon, 2014, 80, 339–350 CrossRef.
- R. A. Sheldon, Chem. Ind., 1992, 903–906 CAS.
- R. A. Sheldon, J. Chem. Technol. Biotechnol., 1997, 68, 381–388 CrossRef CAS.
- M. J. Eckelman, J. B. Zimmerman and P. T. Anastas, J. Ind. Ecol., 2008, 12, 316–328 CrossRef CAS.
- R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC.
- R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437–1451 RSC.
- T. G. Gutowski, M. S. Branham, J. B. Dahmus, A. J. Jones, A. Thiriez and D. P. Sekulic, Environ. Sci. Technol., 2009, 43, 1584–1590 CrossRef CAS PubMed.
- B. Nowack, M. Baalousha, N. Bornhöft, Q. Chaudhry, G. Cornelis, J. Cotterill, A. Gondikas, M. Hassellöv, J. Lead, D. M. Mitrano, F. von der Kammer and T. Wontner-Smith, Environ. Sci.: Nano, 2015, 2, 421–428 RSC.
- E. J. Petersen, D. X. Flores-Cervantes, T. D. Bucheli, L. C. C. Elliott, J. A. Fagan, A. Gogos, S. Hanna, R. Kägi, E. Mansfield, A. R. M. Bustos, D. L. Plata, V. Reipa, P. Westerhoff and M. R. Winchester, Environ. Sci. Technol., 2016, 50, 4587–4605 CrossRef CAS PubMed.
- P. A. Holden, J. L. Gardea-Torresdey, F. Klaessig, R. F. Turco, M. Mortimer, K. Hund-Rinke, E. A. Cohen Hubal, D. Avery, D. Barceló, R. Behra, Y. Cohen, L. Deydier-Stephan, P. L. Ferguson, T. F. Fernandes, B. Herr Harthorn, W. M. Henderson, R. A. Hoke, D. Hristozov, J. M. Johnston, A. B. Kane, L. Kapustka, A. A. Keller, H. S. Lenihan, W. Lovell, C. J. Murphy, R. M. Nisbet, E. J. Petersen, E. R. Salinas, M. Scheringer, M. Sharma, D. E. Speed, Y. Sultan, P. Westerhoff, J. C. White, M. R. Wiesner, E. M. Wong, B. Xing, M. Steele Horan, H. A. Godwin and A. E. Nel, Environ. Sci. Technol., 2016, 50, 6124–6145 CrossRef CAS PubMed.
- P. K. Westerhoff, M. A. Kiser and K. Hristovski, Environ. Eng. Sci., 2013, 30, 109–117 CrossRef CAS.
- Y. Wang, P. Westerhoff and K. D. Hristovski, J. Hazard. Mater., 2012, 201–202, 16–22 CrossRef CAS PubMed.
- M. A. Kiser, P. Westerhoff, T. Benn, Y. Wang, J. Pérez-Rivera and K. Hristovski, Environ. Sci. Technol., 2009, 43, 6757–6763 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9en00322c |
| This journal is © The Royal Society of Chemistry 2019 |
