Open Access Article
Open Access ArticleDietary exposure to silver nitrate compared to two forms of silver nanoparticles in rainbow trout: bioaccumulation potential with minimal physiological effects†
Nathaniel J.
Clark
 ,
David
Boyle
,
Benjamin P.
Eynon
and
Richard D.
Handy
,
David
Boyle
,
Benjamin P.
Eynon
and
Richard D.
Handy
 *
*
School of Biological and Marine Sciences, University of Plymouth, Plymouth, UK. E-mail: r.handy@plymouth.ac.uk
First published on 24th April 2019
Abstract
The trophic transfer of silver to fishes in aquatic food chains is a concern, but little is known about the dietary accumulation of pristine (Ag NPs) and modified (Ag2S NPs) forms of silver-containing nanoparticles. The current study aimed to assess the bioaccumulation potential of these materials following dietary exposure to 100 mg kg−1 Ag as either AgNO3, Ag NPs or Ag2S NPs compared to no added Ag controls. The experiment consisted of a 4 week uptake phase, followed by a further 2 weeks on the control diet (total 6 weeks). Fish were sampled for total Ag analysis (weeks 1–4 and 6), plasma ions, biochemistry and histology. The highest Ag concentrations were in the mid intestine, hind intestine, kidney, liver and gallbladder, regardless of the type of silver exposure. Overall, there was significantly more Ag accumulation from AgNO3 and Ag NP exposure compared to the Ag2S NP treatment, indicating a lower bioavailability of the latter. Following the 4 week exposure, the highest Ag concentrations (from AgNO3, Ag NPs and Ag2S NPs, respectively) was in the hind intestine (140, 90, 0.5 μg g−1), liver (120, 130 and 11 μg g−1) and gallbladder (20, 40 and 1 μg g−1). The liver concentration represented around 40% of the body burden of Ag for all Ag treatments. Following the depuration period (week 6), the Ag concentrations in the tissues showed some transient changes. Notably, there was a significant increase in the liver Ag body burden (66, 63 and 99% for AgNO3, Ag NPs and Ag2S NPs, respectively) in the post-exposure phase. An in chemico digestibility assay simulating low pH in the stomach indicated some dissolution of silver, but there were equal orders of Ag release from both the AgNO3 and Ag NP diets, and none from the Ag2S NPs. There were no treatment-dependent differences in cumulative food intake or intestinal morphology, and only minor transient changes in plasma ions, total glutathione and TBARS in the organs. Overall, the dietary bioaccumulation potential of the nano forms of silver was equal to, or less than the metal salt, and with minimal toxicological effects following 4 weeks exposure.
Environmental significanceLittle is known about the trophic transfer of nano silver to fishes in aquatic food chains and there are concerns about dietary accumulation of pristine silver nanoparticles (Ag NPs) as well as silver sulphide particles (Ag2S NPs) that are likely the most persist form in the environment. Dietary bioaccumulation of Ag from food containing silver nitrate (AgNO3), Ag NPs, and less from food containing Ag2S NPs, was observed. There was limited clearance of the Ag from the internal organs, indicating a bioaccumulation hazard for the materials. Overall, the dietary bioaccumulation potential of the nano forms of silver was equal to, or less than the metal salt, and without overt toxic effects on the fish. |
Introduction
The persistence in the environment, bioaccumulation potential and toxicity (PBT) are key triggers of concern for the environmental risk assessment of chemicals, including engineered nanomaterials (ENMs); but of these processes, bioaccumulation has received little attention (review, Handy et al.1). Dissolved silver is very toxic to aquatic species (review, Ratte2). The uptake of total Ag from food exposures of silver salts have been reported for freshwater fish with dietary bioavailability typically being a few percent of the ingested dose;3,4 in keeping with other studies on dietary metal salts in fish (reviews,5,6). However, less is understood about the dietary uptake and bioaccumulation potential of ENMs in fish.1The ecological concern is that the propensity of ENMs to settle from the water column will lead to exposure of the sediments and benthic species that comprise the base of food webs; leading to subsequent dietary exposure of higher trophic levels including predatory fish in the water column.7–9 This is an issue for Ag-containing ENMs, but pristine Ag NPs can be transformed in the environment to stable, persistent Ag2S NPs.10–12 Indeed, sulfidation is regarded as the primary transformation of Ag NPs during wastewater treatment,13 such that Ag2S NPs are likely the main form of release to the aquatic environment.
There are only a few reports of dietary exposures to ENMs in freshwater fish. For single walled carbon nanotubes14 and TiO2 NPs,15 the dietary bioavailability to the internal organs appears to be small or negligible. However, this is not the case with some other ENMs. For example, Connolly et al.16 fed rainbow trout with food containing 300 or 1000 mg kg−1 ZnO, and after 12 days the intestine and gill tissue concentrations of total Zn were greater than the controls, and the elevated Zn concentrations persisted in those tissues for some weeks during a depuration phase. Interestingly, there were only transient changes in the plasma and liver Zn concentrations.16 Zebrafish (Danio rerio) exposed for 60 days to 0.8% body weight of 4 mg kg−1 of two sizes of cadmium quantum dots (CdS NPs; 8 or 50 nm diameter) showed elevated total Cd concentrations in the livers from both sizes of the material compared to the unexposed controls.17 However, a key concern for risk assessment is whether the nano form is more hazardous than the equivalent concentration of a metal salt. In sea bream (Pagrus major) at least, animals fed diets containing 4 mg kg−1 of Cu as Cu NPs or CuSO4 for 60 days showed similar increases in total Cu concentrations in the whole carcass and liver; although Cu dissolution and particle characterisation was not reported.18
Recently, our laboratory explored the potential bioavailable fraction of total Ag released from fish food using a sequential extraction procedure that simulated the digestion of food in the gut of fish.1 This in chemico digestibility assay showed that both AgNO3 and Ag NPs have an extractable fraction of Ag between 1–4% of the dose, depending on the region of the gut being simulated; and less for Ag2S NPs. This raises the concern that ingested Ag-containing ENMs could be available via the gut to fish and that the hazard will depend on the chemical form of the ENM. The aim of the present study was therefore to explore the in vivo dietary bioaccumulation potential of total Ag from exposures to diets containing AgNO3, Ag NPs or Ag2S NPs compared to unexposed controls for rainbow trout (Oncorhynchus mykiss). In addition, a post-exposure phase was included to inform on the clearance of any apparent Ag accumulation by the fish. The in chemico digestibility assay was also used to assess any apparent dissolved Ag release from the food pellets at the acidic pH of the stomach. Finally, it is also critical to link any apparent bioaccumulation with biological effects, consequently the health of the animals was monitored in terms of food intake and intestinal morphology. Given the known interferences of dissolved Ag with sodium homeostasis, plasma and tissue electrolytes were also measured along with biomarkers of oxidative stress in the tissues (total glutathione, GSH, and thiobarbituric acid reactive substances, TBARS).
Methodology
Experimental design
Juvenile rainbow trout weighing ∼10 g (n = 350) were obtained from Exmoor Fisheries and held for 14 days in a quarantine tank prior to the experiment. Fish were graded and transferred into a flow through systems containing 12 glass tank aquaria (70 L; 26 fish per tank). The water flow rate was 0.52 ± 0.07 L min−1 (n = 12) and did not statistically differ (one way ANOVA, P = 0.328) between treatments. Fish were left in the tanks for two days prior to the experiment to settle and form social hierarchies. Treatments were randomly assigned to tanks (3 tanks per treatment). Fish were fed one of four diets for four weeks: a control diet (no added Ag), or 100 mg kg−1 Ag as AgNO3, Ag NP or Ag2S NPs. Following this, a two-week depuration period occurred where all treatments were fed the control diet. Fish were fed a 2% body weight ration per day whereby the amount of food was altered each week based on the tank biomass. Fish were fed carefully at approximately 9 am, 1 pm and 5 pm each day and observed to ensure the daily ration was eaten immediately. There were no residual food pellets in the water after each feed. The photoperiod was set to 12 h light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h dark. The tanks were also cleaned periodically, with careful siphoning of any residual faecal matter from the tanks to ensure water quality.
12 h dark. The tanks were also cleaned periodically, with careful siphoning of any residual faecal matter from the tanks to ensure water quality.
Water samples were taken daily for oxygen, temperature, pH and ammonia. Water samples for measuring total silver concentration were collected once a week. There was no consistent detectable Ag in the control or Ag2S NP treatments (<0.10 ng mL−1; LOD). Despite monitoring the cleanliness of the fish tanks, the AgNO3 and Ag NP treatments showed a trace amount of total silver in the tanks that was just above the LOD of 0.40 ± 0.04 and 0.47 ± 0.04 ng mL−1 (mean ± S.E.M., n = 24 per treatment), respectively. No Ag was detected during the depuration period.
Six of the stock fish were sampled at the start of the experiment for reference and to determine the background tissue Ag concentrations in the animals. Subsequently, fish from the experimental tanks were sampled at weeks 1, 2, 3 and 4 during the exposure phase and at the end of the depuration phase on clean food (week 6) for trace metal analysis (i.e., at least 2 fish per tank equating to 6 fish per treatment at each time point). Samples for biochemistry and histology were taken at week 4. Blood samples were also collected at weeks 2, 4 and 6 (see below). The entire experiment was conducted with ethical approval from the UK Home Office via a Project Licence held at Plymouth University under the Animals (Scientific Procedures) Act (1986), and its amendments, in compliance with Directive 2010/63/EU. In order to facilitate dissection and to ensure animal welfare during anaesthesia (see below), fish were not fed on the morning of the sampling days. The last feed of the fish was the evening before sampling, allowing time for evacuation of the gut contents (∼15 h).
Nanoparticles and diet formulation
The ENMs were supplied as part of the EU NanoFase project, and the characterisation of batches of the same materials used here are reported elsewhere.19 Briefly, the Ag NPs and Ag2S NPs were provided by Applied Nanoparticles (Barcelona). The Ag NPs were supplied (manufacture's information) at a nominal size and concentration of 50 nm and 10.4 g L−1, respectively. The Ag2S NPs had a nominal size and concentration of 20 nm and 9.6 g L−1, respectively. The Ag NPs were dispersed in 25 μM tannic acid and 5.5 mM sodium citrate, and 1 mg mL−1 polyvinylpyrrolidone (PVP), and the Ag2S NPs were dispersed in 1 mg mL−1 PVP only. Transmission electron microscopy (TEM, JEOL-1200EX II) was conducted at Plymouth to confirm the primary particle diameters of the materials. Briefly, a copper grid was placed on top of a drop of the stock suspensions; after which the grid was removed and allowed to dry for 10 min at room temperature before imaging. The TEM revealed the primary particle diameter of Ag NPs and Ag2S NPs to be 55 ± 3 nm (mean ± S.D., n = 120) and 37 ± 19 nm (mean ± S.D., n = 103), respectively (as reported in Clark et al.20). The concentration of the total Ag in the stocks of Ag NPs and Ag2S NPs was also measured by inductively coupled plasma mass spectrometry (ICP-MS, Thermo X-series 2 ICP-MS). The stock concentrations for (mean ± S.D., n = 4) the Ag NPs and Ag2S NPs were 9.5 ± 0.4 and 1.4 ± 0.1 g L−1.The diet used throughout the study was a commercial fish food (Aller Futura, EX, Kaliningrad, Russia), with a pellet size of 1.5 mm. The proximate composition of the diets was (% dry weight from manufacturer's guidelines): lipid, 17; protein, 58; ash, 10.1; fibre, 0.9. The intact food pellets were supplemented with a stock dispersion of the relevant nanomaterial or AgNO3 that was allowed to soak into the pellets and this was then sealed with a topcoat of 10% gelatine; similar to the method used in our previous dietary studies on ENMs.21 The dosing dispersions for mixing with the diets were prepared by sonicating (FB15048 ultrasonic bath, 35 W, Thermo Fisher) 100 mL of a nominal stock concentration of 1 g L−1 of Ag as either AgNO3, Ag NPs and Ag2S NPs prepared in ultrapure water for 1 h (i.e., a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dilution of the stock supplied by the manufacturer). Nanoparticle tracking analysis (NTA) was conducted to confirm the materials could be dispersed adequately in these stocks. The mean (± S.D.) hydrodynamic diameter was 73 ± 2 nm (n = 3) for 1 mg L−1 Ag NPs and a mean hydrodynamic diameter of 124 ± 31 nm (n = 3) for 1 mg L−1 Ag2S NPs. This was slowly added to 900 g of the diet and thoroughly, but gently mixed with a commercial food mixer (Kenwood KM810/KM816, 2004). A solution of 10 g of porcine gelatine (>98% purity, Sigma-Aldrich) in 100 mL of ultrapure water was prepared by gentle heating to 40 °C, allowed to cool for 10 minutes, and then gently poured over the diet and mixed in for 15 minutes. The unexposed control diet was prepared in exactly the same way, but dosed with ultrapure water without any silver. The diets were then placed in an incubator at 45 °C and left to dry overnight. The expected nominal concentration in the silver-supplemented diets was 100 mg of total Ag kg-1 dry weight of food. The total Ag concentrations of the diets were measured by ICP-MS (see below) with 0.72 ± 0.22, 92.35 ± 7.33, 96.19 ± 11.49 and 88.16 ± 23.16 mg kg−1 dry weight (mean ± S.E.M., n = 8) in the control, AgNO3, Ag NP and Ag2S NP treatments respectively. There was no significant difference between the Ag concentrations in the exposure diets (one way ANOVA, P > 0.05).
10 dilution of the stock supplied by the manufacturer). Nanoparticle tracking analysis (NTA) was conducted to confirm the materials could be dispersed adequately in these stocks. The mean (± S.D.) hydrodynamic diameter was 73 ± 2 nm (n = 3) for 1 mg L−1 Ag NPs and a mean hydrodynamic diameter of 124 ± 31 nm (n = 3) for 1 mg L−1 Ag2S NPs. This was slowly added to 900 g of the diet and thoroughly, but gently mixed with a commercial food mixer (Kenwood KM810/KM816, 2004). A solution of 10 g of porcine gelatine (>98% purity, Sigma-Aldrich) in 100 mL of ultrapure water was prepared by gentle heating to 40 °C, allowed to cool for 10 minutes, and then gently poured over the diet and mixed in for 15 minutes. The unexposed control diet was prepared in exactly the same way, but dosed with ultrapure water without any silver. The diets were then placed in an incubator at 45 °C and left to dry overnight. The expected nominal concentration in the silver-supplemented diets was 100 mg of total Ag kg-1 dry weight of food. The total Ag concentrations of the diets were measured by ICP-MS (see below) with 0.72 ± 0.22, 92.35 ± 7.33, 96.19 ± 11.49 and 88.16 ± 23.16 mg kg−1 dry weight (mean ± S.E.M., n = 8) in the control, AgNO3, Ag NP and Ag2S NP treatments respectively. There was no significant difference between the Ag concentrations in the exposure diets (one way ANOVA, P > 0.05).
Tissue collections and blood sampling
Blood sampling and dissection followed Shaw et al.22 The experimental fish were blood sampled at weeks 2–6 (the animals were initially too small to collect blood before week 2). For blood sampling, (n = 2 fish per tank, n = 6 per treatment) were anaesthetised using buffered (NaHCO3) MS222, pithed (to destroy the brain) and the whole blood removed via the caudal vein into heparinised syringes. Then 50 μL of whole blood was digested in 0.2 mL of concentrated nitric acid at 60 °C for 4 hours, allowed to cool and diluted to 2 mL with ultrapure water prior to analysis for total Ag by ICP-MS (see below). The remaining whole blood was centrifuged, and the plasma removed and stored at −20 °C until required. The plasma Na+ and K+ concentrations were analysed by flame photometry (Sherwood Model 420 Flame Photometer). The fish were then dissected for biochemical analysis (see below).For metal analysis, the fish were dissected for the mid intestine, hind intestine, liver (following gallbladder removal), kidney, spleen, gill and brain (n = 2 fish per tank, n = 6 per treatment). The mid and hind intestine were rinsed in ultrapure water and blotted before wet weight determination. Care was taken to avoid cross-contamination between fish with clean, acid-washed instruments. Tissues (0.001–0.363 g) and carcasses (7–38 g) were freeze dried (Lablyo freeze dryer) for 24 h and weighed. Once dried, the hind intestine, kidney and liver, of week 2, 4 and 6 only, were cut in half; one half was used for total Ag concentrations (and electrolytes) reported here, and the other half for particulate analysis (reported elsewhere). The freeze drying was primarily to dry the samples, but the process also enabled the carcass samples to be crushed to a powder so that a sub-sample of carcass could then be used for acid digestion and total Ag determination (see below).
Trace metal analysis
Tissue metal analysis was similar to Shaw et al.22 with modifications for silver. Tissues were freeze dried, weighed and digested using 200 μL of analytical grade (primer plus) nitric acid and heated using a water bath to 60 °C for 4 h. With every analysis, procedural blanks were analysed to check for leaching from the test tubes or other incidental contamination from the reagents (not observed, blanks remained below the limit of detection of the instrument). Following digestion, the samples were diluted to 2 mL using 22.2 μg L−1 indium and iridium spiked ultrapure water (for use as internal standards) before being analysed by ICP-MS. Drift was monitored using the indium and iridium signals and standards were checked after every 15 samples. The samples were matrix-matched with the standards. The 107Ag isotope signal was used to determine total Ag concentration. However, both 107Ag and 109Ag isotopes were measured to aid interpretation of results from values near the limit of detection of the ICP-MS. The limit of detection of the ICP-MS ranged between 0.04 and 0.18 ng mL−1 depending on the sample type, which equates to between 1 and 25 ng g−1 tissue dw. In addition to Ag determination, tissue samples from week 4 (the end of the exposure phase) were diluted (0.5 mL sample diluted to 2.5 mL) and analysed by ICP-OES for the tissue electrolyte composition (Cu, Fe, Mn, Na, Ca and K) according to Shaw et al.22Histology examination
Fish were sampled at week 4 for histological examination according to Al-Bairuty et al.23 Animals were randomly selected (n = 2 per tank/n = 6 per treatment), euthanized (as above) and carefully dissected to collect the second gill arch, mid intestine, hind intestine and liver. Tissues were immediately placed in 10% buffered formal saline for at least one week for fixation. Tissues were processed using an automated tissue processor (Leica TP1020 semi-enclosed benchtop) where samples were taken from the formal saline into industrial methylated spirit (50–100%), followed by clearing using histolene and then taken to wax (∼20 h total time). Tissues were then embedded in wax blocks (Leica EG 1150H) and sectioned at 6 μm intervals (Leica RM2235 microtome) and dried overnight. For the mid and hind intestinal morphology, slides were stained with 0.5% haematoxylin and counter-stained with 1.1% van Gieson's to differentiate the connective tissues, followed by 1.0% alcian blue for the mucocytes in the epithelium. Mallory's trichrome was used for gill morphology. Tissues were viewed and photographed using a Leica microscope (DMD108) with a built in camera. The dimensions of the mucocytes were measured manually using the embedded software in the Leica microscope.Biochemistry
The mid intestine, hind intestine, liver, kidney, gill and brain were sampled at week 4 for biomarkers of oxidative stress including the TBARS assay and total GSH according to Smith et al.24 Following dissection, tissues were snap frozen in liquid nitrogen and stored at −80 °C until required for analysis. Tissues (approximately 0.2 g) or the whole brain were thawed on ice and homogenised (Cat X520D with a T6 shaft, medium speed, Bennett & Co., Weston-Super-Mare) into 1 mL of ice-cold isotonic buffer [in mmol L−1; 300 sucrose, 0.1 ethylenediaminetetraacetic acid (EDTA), 20 (4-(2-hydroxyethyl)piperazine-1-ethane sulfonic acid (HEPES)), adjusted to pH 7.8 with a few drops of tris (2-amino-2-hydroxymethyl-1,3-propanediol)]. Following centrifugation (2 min at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm), the supernatant from the crude tissue homogenates were stored at −80 °C until required. The samples were analysed (in triplicate) for TBARS and total GSH.
000 rpm), the supernatant from the crude tissue homogenates were stored at −80 °C until required. The samples were analysed (in triplicate) for TBARS and total GSH.
For the TBARS assay, 130 μL of sample or standard was added to an Eppendorf tube followed by 32.5 μL of 1 mmol L−1 butylated hydroxytoluene (2,6-di-O-tert-butyl-4-methylphenol or BHT) to prevent oxidation, 455 μL of phosphate buffer (100 mmol L−1 potassium phosphate and 5 mM EDTA) and then 162.5 μL of 50% (w/v) trichloroacetic acid (TCA) to precipitate excess protein. The samples were then centrifuged for 2 min at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. Following this, 150 μL of the supernatant was added to 3 wells in a 96-well plate, followed by the addition of 75 μL of 1.3% TBA (in 0.3% NaOH). The plate was covered and incubated for 60 min at 60 °C, allowed to cool to room temperature and the absorbances measured at 530 nm against standards (0.5–25 nmol mL−1 1,1,3,3-tetraethoxypropane).
000 rpm. Following this, 150 μL of the supernatant was added to 3 wells in a 96-well plate, followed by the addition of 75 μL of 1.3% TBA (in 0.3% NaOH). The plate was covered and incubated for 60 min at 60 °C, allowed to cool to room temperature and the absorbances measured at 530 nm against standards (0.5–25 nmol mL−1 1,1,3,3-tetraethoxypropane).
For total glutathione, 20 μL of sample homogenate and 140 μL of mixture containing 0.714 mmol L−1 DTNB (5,5′-dithiobis(2-nitrobenzoic acid)), 100 mmol L−1 phosphate buffer and 0.357 U mL−1 glutathione reductase into a 96-well plate. To start the reaction, 40 μL of 1 mmol L−1 NADPH was added to each well. The rate of change of the absorbance of each sample were read at 412 nm for 15 min with readings every 15 seconds, and compared to GSH standards. TBARS and GSH were normalised to the protein concentration of each homogenate. Protein was determined in triplicate using 25 μL of sample using a commercially available kit (Pierce BCA protein assay kit 23227, Thermo Fisher). To check for interferences, the assays were run with the appropriate amount of Ag spiked into the samples at their respective tissue concentrations of total Ag. No interferences were found.
In chemico digestibility
To aid data interpretation over the bioavailability and form of Ag in the gut lumen of rainbow trout, an in chemico digestibility assay was used.1 Two compartments of gastrointestinal tract were simulated with artificial solutions: the stomach (0.1 M HCl, 0.9% NaCl, pH 2) and the intestine (0.9% NaCl, pH 7.8). The experimental diets (described above) were placed into 15 cm sections of clean dialysis tubing (typical pore size <2 nm) and tied closed (1 g food per bag, n = 4 replicates). Samples were then incubated in 20 mL of the respective artificial gut fluid for 4 h in rotating tubes at 30 rpm (Stuart Tube Roller SRT6) to mimic peristalsis movements. Afterwards, the external fluid was collected for total Ag determination by ICP-MS (as above).Calculations and statistics
For growth performance in each tank, the average body weight of the fish in each tank was calculated each week as the total biomass (g)/number of fish per tank. In order to derive the body distribution of silver amongst the organs of the fish the absolute amount of total Ag in each organ was first calculated and then expressed as a percentage of the body burden. The absolute mass of Ag in the organ was calculated from the measured concentration in the organ (μg g−1 dry weight) multiplied by the dry weight of the whole organ. In order to determine the whole body burden, the absolute mass of Ag in each of the organs was added together, plus that of the remaining carcass. Values for each organ were expressed as a percentage of the total body burden. All data analysis was performed in SigmaPlot 13.0. Data were checked for outliers using Grubb's test, following which they were assessed for normality (Shapiro–Wilk test) and equal variance (Brown Forsythe). Statistical differences were assessed using either a one-way ANOVA (growth, cumulative feed, tissue moisture and electrolyte composition, mucocyte size, biochemical endpoints) or two-way ANOVA for analysis treatment and time (tissue Ag concentrations, Ag body distribution and in chemico digestibility). For non-parametric data, log10 transformations were performed, or followed by a Kruskal–Wallis test as appropriate. P values presented are from Holm–Sidak for parametric data and either Dunn's test or Student–Newman–Keuls test for non-parametric data.Results
Tissue total Ag concentration in the organs
The initial stock fish were analysed for total Ag by ICP-MS and showed trace amount of Ag in the liver tissue only (0.43 ± 0.06 μg g−1 dw Ag, mean ± S.E.M., n = 6). The remaining tissues had Ag concentrations that were below the limit of detection of the instrument (<50 ng g−1 dw). Over the 6 week experiment, the organs of the unexposed control fish; including the spleen, brain, gills, gallbladder, kidney, mid intestine and carcass, had no detectable total Ag concentration. Trace amounts of Ag were found in the hind intestine and liver of the unexposed fish, which remained low but with some transient time-dependent changes (Table 1).| Tissue | Week 1 | Week 2 | Week 3 | Week 4 | Week 6 |
|---|---|---|---|---|---|
| Data are means ± S.E.M., n = 5/6. Limit of detection (LOD) of organs ranged from 0.8 to 25 ng g−1 dry weight. The LOD in whole blood samples was 5.2 ng mL−1. N/A indicates no sampling at that time point occurred. Different upper case letter denotes statistical difference between treatments within the same week (i.e., treatment effect within tissue by columns). Different lower case letters denotes statistical difference between weeks within the same treatment (i.e., time-effect within rows). Data were analysed using a two-way ANOVA or Kruskal–Wallis. Note, the exposure phase was until the end of week 4, followed by two further weeks on normal food to week 6. | |||||
| Mid intestine | |||||
| Control | <LOD | <LOD | <LOD | <LOD | <LOD |
| AgNO3 | 6.33 ± 1.80Aab | 8.31 ± 1.04Aab | 11.86 ± 2.52Aa | 12.77 ± 3.30Aab | 4.79 ± 1.72Ab |
| Ag NPs | 3.24 ± 0.51Aa | 5.65 ± 1.15Aab | 12.48 ± 3.13Ab | 10.08 ± 1.60Aab | 12.19 ± 4.15Aab |
| Ag2S NPs | 0.24 ± 0.04Ba | 0.17 ± 0.03Ba | 0.22 ± 0.05Ba | 0.21 ± 0.05Ba | <LOD |
| Hind intestine | |||||
| Control | 0.05 ± 0.01Aa | 0.04 ± 0.01Aa | 0.04 ± 0.00Aa | 0.02 ± 0.01Aa | 0.04 ± 0.01Aa |
| AgNO3 | 61.38 ± 6.12Bab | 62.35 ± 6.62Bab | 85.59 ± 10.37Bab | 140.02 ± 22.38Ba | 52.88 ± 10.55Bb |
| Ag NPs | 35.55 ± 5.20Ba | 65.15 ± 3.19Ba | 70.43 ± 7.16Ba | 89.87 ± 13.61Ba | 46.36 ± 8.19Ba |
| Ag2S NPs | 1.92 ± 0.29Ca | 1.15 ± 0.22Ca | 1.12 ± 0.26Ca | 0.46 ± 0.09Cb | 0.04 ± 0.01Ac |
| Liver | |||||
| Control | 0.28 ± 0.02Aab | 0.23 ± 0.01Aa | 0.45 ± 0.06Ab | 0.44 ± 0.03Ab | 0.53 ± 0.06Ac |
| AgNO3 | 7.27 ± 1.02Ba | 79.76 ± 11.52Bb | 89.43 ± 5.93Bb | 121.77 ± 9.58Bb | 113.33 ± 23.66Bb |
| Ag NPs | 5.65 ± 0.57Ba | 64.50 ± 6.40Bb | 85.03 ± 11.86Bbc | 128.63 ± 17.41Bc | 87.67 ± 4.08Bbc |
| Ag2S NPs | 1.98 ± 0.31Ca | 4.46 ± 0.68Cb | 7.71 ± 0.94Cc | 10.93 ± 0.95Cc | 10.41 ± 2.15Cc |
| Gallbladder | |||||
| Control | <LOD | <LOD | <LOD | <LOD | <LOD |
| AgNO3 | 10.60 ± 3.18Aa | 34.08 ± 9.18Aa | 20.66 ± 3.56Aa | 19.13 ± 3.25Aa | 6.62 ± 2.31Aa |
| Ag NPs | 15.60 ± 4.11Aa | 14.64 ± 1.69Aa | 22.84 ± 4.97Aa | 39.79 ± 14.41Aa | 10.37 ± 4.02Aa |
| Ag2S NPs | 0.17 ± 0.06Ba | 0.64 ± 0.11Ba | 1.01 ± 0.20Ba | 0.94 ± 0.33Ba | 0.26 ± 0.13Ba |
| Kidney | |||||
| Control | <LOD | <LOD | <LOD | <LOD | <LOD |
| AgNO3 | 2.17 ± 0.31Aa | 8.68 ± 1.54Abc | 8.32 ± 1.07Ab | 23.59 ± 3.76Abc | 24.77 ± 4.95Ac |
| Ag NPs | 3.24 ± 0.75Aa | 7.16 ± 1.80Aab | 8.87 ± 1.95Abc | 23.01 ± 6.59Acd | 31.16 ± 8.05Ad |
| Ag2S NPs | 0.12 ± 0.04Ba | 0.19 ± 0.04Bab | 0.76 ± 0.26Bb | 0.37 ± 0.11Bb | 0.14 ± 0.06Ba |
| Spleen | |||||
| Control | <LOD | <LOD | <LOD | <LOD | <LOD |
| AgNO3 | 0.28 ± 0.05Aa | 0.54 ± 0.09Aab | 0.68 ± 0.07Aab | 1.24 ± 0.21Ab | 0.64 ± 0.10Aab |
| Ag NPs | 0.24 ± 0.00Aa | 0.49 ± 0.11Aa | 0.71 ± 0.17Aab | 1.94 ± 0.57Ab | 2.31 ± 0.80Bb |
| Ag2S NPs | <LOD | <LOD | <LOD | <LOD | <LOD |
| Gill | |||||
| Control | <LOD | <LOD | <LOD | <LOD | <LOD |
| AgNO3 | 0.73 ± 0.32Aa | 0.75 ± 0.12Aa | 0.83 ± 0.11Aa | 1.30 ± 0.28Aa | 0.35 ± 0.09Aa |
| Ag NPs | 0.55 ± 0.06Aa | 0.63 ± 0.10Aa | 0.92 ± 0.12Aa | 1.66 ± 0.30Aa | 0.63 ± 0.08Aa |
| Ag2S NPs | 0.14 ± 0.06Bab | 0.05 ± 0.01Ba | 0.77 ± 0.43Bb | 0.03 ± 0.01Ba | <LOD |
| Brain | |||||
| Control | <LOD | <LOD | <LOD | <LOD | <LOD |
| AgNO3 | 0.46 ± 0.06Aa | 0.84 ± 0.12Aab | 1.08 ± 0.19Abc | 1.53 ± 0.13Ac | 1.08 ± 0.20Abc |
| Ag NPs | 0.43 ± 0.06Aa | 0.57 ± 0.05Aab | 1.02 ± 0.11Abc | 1.57 ± 0.28Ac | 1.05 ± 0.14Abc |
| Ag2S NPs | <LOD | 0.04 ± 0.00Ba | 0.07 ± 0.02Ba | 0.02 ± 0.00Ba | 0.04 ± 0.01Ba |
| Carcass | |||||
| Control | <LOD | <LOD | <LOD | <LOD | <LOD |
| AgNO3 | 1.01 ± 0.21Aa | 1.11 ± 0.17Aa | 1.19 ± 0.14Aa | 2.02 ± 0.35Aa | 0.17 ± 0.02Ab |
| Ag NPs | 1.21 ± 0.42Aa | 1.01 ± 0.17Aa | 1.33 ± 0.23Aa | 2.05 ± 0.23Aa | 0.26 ± 0.07Ab |
| Ag2S NPs | 0.08 ± 0.03Ba | 0.16 ± 0.05Ba | 0.26 ± 0.08Ba | 0.17 ± 0.04Ba | <LOD |
| Blood | |||||
| Control | N/A | <LOD | N/A | <LOD | <LOD |
| AgNO3 | N/A | 249.76 ± 29.71Aa | N/A | 268.59 ± 17.28Aa | 36.93 ± 4.18Aa |
| Ag NPs | N/A | 225.33 ± 13.70Aa | N/A | 260.61 ± 20.97Aa | 54.97 ± 5.74Aa |
| Ag2S NPs | N/A | 9.57 ± 2.21Ba | N/A | 21.39 ± 11.78Ba | <LOD |
During the 4 week exposure phase to dietary AgNO3, there were elevated total Ag concentrations in all the organs compared to the unexposed controls, with persistent increases of total Ag concentrations found in the mid and hind intestine in keeping with the route of exposure. The liver as a central compartment in metal accumulation also showed a gradual elevation of Ag with the highest total Ag concentration at week 4 of AgNO3 diet compared to week 1 (two-way ANOVA; P < 0.001). There were also especially elevated Ag concentrations in the gallbladder, whole blood and kidney of the AgNO3 treatment compared to the unexposed controls (Table 1). In contrast, the gills of fish fed the AgNO3 diet showed a steady total Ag concentration of around 1 μg g−1 dw or less, in keeping with the organ being perfused with Ag-containing blood rather than incidental Ag exposure via the water. Two-way ANOVA's revealed the brain (P < 0.05) and spleen (P < 0.05) had time-dependent increases in total Ag, but these did not exceed 2 μg g−1 dw by the end of the exposure phase. The whole blood Ag concentrations remained constant throughout the exposure phase (two-way ANOVA; P > 0.05).
The AgNO3 and Ag NP treatments showed a very similar profile of total Ag concentrations in the organs and whole blood, with no statistically significant differences between these two treatments over the 4 weeks (Table 1). For example, the livers of fish from both treatments at week 4 showed total Ag concentrations around 122–129 μg g−1 dw. However, both the AgNO3 and Ag NP treatments showed statistically significantly higher total Ag concentrations compared to the Ag2S NP treatments in all tissues from week 1 to 4 (Table 1). For example, the livers (two-way ANOVA; P < 0.001) of fish from the Ag2S NP treatment showed only 11 μg g−1 dw of total Ag; an order of magnitude less Ag than either the Ag NP or AgNO3 dietary treatments. Crucially, the mid (P < 0.001) and hind (P < 0.001) intestine of fish from the Ag2S NP treatment accumulated only 2 μg g−1 dw of total Ag or much less (two-way ANOVA; Table 1), which was 10–100 fold less than the intestine of the other Ag treatments and indicating that Ag from Ag2S form was the least available for uptake by the gut. Within the Ag2S NP treatment, there were no time-dependent changes in the gallbladder, brain or carcass concentration (P > 0.05), with values of around 1 μg g−1 dw or less; although there were some transient increases in the total Ag in the kidney (P < 0.03) and hind intestine (P < 0.001) during the exposure to the Ag2S diet (two-way ANOVA; Table 1).
After the 4 weeks of exposure to the Ag-containing diets, there was a two-week depuration phase where the fish from all treatments were fed the unexposed control diet. In the animals previously fed the AgNO3 diet, a two-way ANOVA revealed there were statistically significant decreases in the total Ag concentrations in the hind intestine (P < 0.001) and carcass (P < 0.001) at the end of the depuration phase compared to the values at the end of the exposure phase (week 4, Table 1). However, there was no appreciable clearance of Ag from the other internal organs of fish from the AgNO3 treatment, including the liver (Table 1). An identical pattern of decreasing, or unaltered, total Ag concentrations were found in the organs of fish from the Ag NP treatment in the post exposure phase. Despite some evidence of clearance, none of the organs that had shown elevated total Ag concentrations recovered to control levels, with at least one third or more of the total Ag remaining in the organs of fish from both the AgNO3 and Ag NP treatments (Table 1). The Ag2S treatment showed a similar pattern of decreasing total Ag concentrations in the organs in the post exposure phase, but with a few crucial differences. Unlike the other treatments, the kidney (P = 0.029) showed statistically significant decreases in the total Ag concentrations at week 6 (two-way ANOVA); and in the case of the gill, carcass and the blood, the total Ag returned to control levels (below the detection limit); likely because these compartments had only accumulated a small amount of total Ag in the first place.
Body burden and distribution of Ag for AgNO3, Ag NP and Ag2S NP treatments
The calculated body distribution in the animals at the end of the exposure and the end of the depuration phase are shown (Table 2). The Ag body distribution for the control treatment was not calculated because several organs were below the limit of detection. At the end of the 4 week exposure, the Ag body distribution in the AgNO3 treatment was as expected with the organs containing a percentage of body burden in the following order: carcass > liver > hind intestine > kidney > gallbladder > mid intestine > gill > brain > spleen. Critically, the liver contained around two fifths of the body burden. For the Ag NP treatment, the profile of the body burden was similar to the AgNO3 treatment and in the order: carcass > liver > hind intestine > kidney > gallbladder > mid intestine > gill > brain > spleen. There was no significant difference between the body burden of any organ in the AgNO3 and Ag NP treatments (two-way ANOVA or Kruskal–Wallis; P > 0.05). Small changes were found in the order of the distribution of the body burden in the Ag2S NP treatment with: carcass > liver > kidney > hind intestine > gallbladder > gill > mid intestine > brain > spleen. However, the organs that accounted for the majority of the body burden remained the carcass, liver, kidney and hind intestine (>99% of the total), similar to the AgNO3 and Ag NP treatments (98 and 97% of the total, respectively).| Tissue | Treatment | Week 4 | Week 6 | ||
|---|---|---|---|---|---|
| Amount Ag (ng) | % body burden | Amount Ag (ng) | % body burden | ||
| Data are mean ± S.E.M. (n = 5/6). Blood Ag was not included in body distribution % (not taken from the same individual fish so not valid comparison). Control fish are excluded for clarity due to small signals in the hind intestine and liver tissues. Note, the exposure phase was until the end of week 4, followed by two further weeks on normal food to week 6. Statistical analysis was only performed on the % body burden (two-way ANOVA or Kruskal–Wallis) for treatment and time. Different upper case letter denotes statistical difference between treatments within the same week (i.e., treatment effect within tissue by columns). Different lower case letters denotes statistical difference between weeks within the same treatment (i.e., time-effect within rows). Statistical analysis not performed on the mass of Ag in the tissue as this is not standardised data with respect to organ weight. | |||||
| Mid intestine | AgNO3 | 126.1 ± 46.6 | 0.47 ± 0.18Aa | 188.5 ± 77.5 | 1.51 ± 0.38Aa |
| Ag NPs | 81.6 ± 18.0 | 0.35 ± 0.04Aa | 266.4 ± 85.4 | 2.30 ± 0.81Aa | |
| Ag2S NPs | 2.2 ± 0.7 | 0.13 ± 0.05Ba | 0.2 ± 0.0 | 0.02 ± 0.00Bb | |
| Hind intestine | AgNO3 | 2022.7 ± 760.8 | 9.80 ± 3.98Aa | 1591.1 ± 213.2 | 11.52 ± 2.59Aa |
| Ag NPs | 1295.7 ± 413.4 | 5.27 ± 1.31Aa | 1601.8 ± 275.8 | 12.20 ± 2.16Aa | |
| Ag2S NPs | 17.4 ± 9.6 | 0.90 ± 0.37Ba | 1.8 ± 0.6 | 0.17 ± 0.03Bb | |
| Liver | AgNO3 | 7343.5 ± 515.5 | 38.25 ± 3.05Aa | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615.4 ± 2407.4 615.4 ± 2407.4 |
65.90 ± 5.10Ab |
| Ag NPs | 8327.7 ± 947.5 | 38.38 ± 4.07Aa | 9200.4 ± 1033.8 | 62.89 ± 2.16Ab | |
| Ag2S NPs | 907.2 ± 68.4 | 44.44 ± 5.34Aa | 937.8 ± 180.2 | 98.88 ± 0.37Bb | |
| Gallbladder | AgNO3 | 137.8 ± 68.7 | 0.84 ± 0.50Aa | 78.7 ± 32.7 | 0.51 ± 0.16Aa |
| Ag NPs | 194.9 ± 20.0 | 0.95 ± 0.18Aa | 88.6 ± 28.1 | 0.64 ± 0.22Aa | |
| Ag2S NPs | 10.5 ± 4.7 | 0.59 ± 0.34Aa | 4.6 ± 3.4 | 0.39 ± 0.26Aa | |
| Kidney | AgNO3 | 760.9 ± 132.9 | 3.88 ± 0.62Aa | 1096.8 ± 266.3 | 9.01 ± 1.63Ab |
| Ag NPs | 966.1 ± 190.1 | 4.16 ± 0.65Aa | 1517.1 ± 317.5 | 9.25 ± 0.90Ab | |
| Ag2S NPs | 59.2 ± 45.3 | 3.11 ± 2.16Aa | 5.4 ± 2.3 | 0.46 ± 0.12Ba | |
| Spleen | AgNO3 | 8.2 ± 1.5 | 0.04 ± 0.01Aa | 5.4 ± 1.0 | 0.05 ± 0.01Aa |
| Ag NPs | 10.6 ± 4.1 | 0.04 ± 0.01Aa | 16.7 ± 5.5 | 0.09 ± 0.02Aa | |
| Ag2S NPs | <LOD | 0.00 ± 0.00 | <LOD | <LOD | |
| Gill | AgNO3 | 67.9 ± 29.6 | 0.33 ± 0.12Aa | 12.8 ± 2.4 | 0.11 ± 0.02Aa |
| Ag NPs | 89.5 ± 45.3 | 0.34 ± 0.14Aa | 32.5 ± 12.1 | 0.28 ± 0.15Aa | |
| Ag2S NPs | 3.7 ± 2.7 | 0.19 ± 0.13A | <LOD | <LOD | |
| Brain | AgNO3 | 21.4 ± 2.5 | 0.11 ± 0.01Aa | 22.0 ± 4.4 | 0.19 ± 0.03Aa |
| Ag NPs | 25.2 ± 6.6 | 0.11 ± 0.02Aa | 18.3 ± 1.9 | 0.13 ± 0.02Aa | |
| Ag2S NPs | 0.9 ± 0.1 | 0.05 ± 0.01Ba | 0.7 ± 0.1 | 0.09 ± 0.02Ab | |
| Carcass | AgNO3 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 606.8 ± 2053.5 606.8 ± 2053.5 |
46.28 ± 3.77Aa | 1442.0 ± 256.9 | 11.21 ± 1.48Ab |
| Ag NPs | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 663.8 ± 1739.9 663.8 ± 1739.9 |
50.41 ± 2.80Aa | 1968.2 ± 378.6 | 12.23 ± 1.20Ab | |
| Ag2S NPs | 942.1 ± 225.7 | 50.58 ± 4.92A | <LOD | <LOD | |
After the depuration period (week 6), there was some evidence of redistribution of the body burden in the AgNO3 treatment (Table 2). For example, there was a 4-fold reduction in the proportion of the body burden associated with the carcass (two-way ANOVA; P < 0.001); this was complemented by a significant rise in the kidney (2.4-fold; P = 0.003) and liver (1.7-fold; P < 0.001). The predominant organ of Ag contamination became the liver (66% of the body burden). This pattern of re-distribution of the body burden from the carcass to the kidney and liver was also observed in the Ag NP and Ag2S NP treatments.
Growth, food intake and mortality
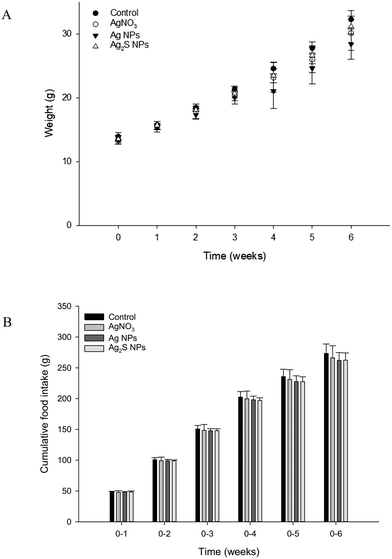
The fish weighed around 10 g at the start of the experiment and growth was steady with around a 4-fold increase in weight over the 6 week period. At the start of each week, there was no significant difference (one-way ANOVA's) between the average fish weight per treatment (P > 0.05), or the weekly cumulative food intake (P > 0.05; Fig. 1). Over the course of the study, there was a total loss of seven fish. Due to the mortalities being spread over five tanks across all treatment, and the presence of fin lesions from observable bullying (a normal aggressive behaviour of trout); these were not attributed to treatment related effects.Blood plasma and tissue electrolyte concentration
The plasma Na+ and K+ concentration were measured from all treatments (Table S1†). The plasma Na+ showed a transient decrease at week 4 in the AgNO3 treatment, but this was close to the normal physiological range, and overall there was no statistically significant differences from the other treatments (P > 0.05). There were some time and treatment related effects in the plasma K+ concentrations (two-way ANOVA; Table S1†), but these were also transient and within the normal range. For example, after 4 weeks of exposure, the plasma K+ concentrations in the Ag2S NP treatment was significantly elevated to 2.8 mmol L−1 compared to only 1.8 mmol L−1 in the control (P = 0.014). However, overall there was no nanomaterial-type effects on either Na+ or K+ concentrations, and after the depuration period there was no significant differences between treatments (two-way ANOVA; Na+, P > 0.05, K+, P > 0.05).The tissue electrolyte concentrations showed only small transient changes between treatments (one-way ANOVA or Kruskal–Wallis; Table S2†). For example, the Na+ concentration in the hind intestine of the AgNO3 treatment was significantly lower (1.83 mg g−1) compared to that for the control (3.13 mg g−1; P = 0.032), Ag NP (3.59 mg g−1; P = 0.004) and Ag2S NP treatments (2.99 mg g−1; P = 0.041). There was no nanomaterial-type effects. The moisture content of the tissue (data not shown) ranged between 69 and 84%, depending on the type of tissue. There was no significant difference between the treatments (one-way ANOVA or Kruskal–Wallis; P > 0.05).
Histological examination and biochemical alterations
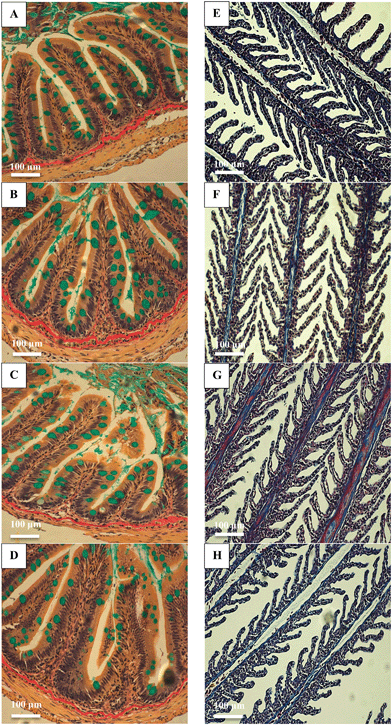
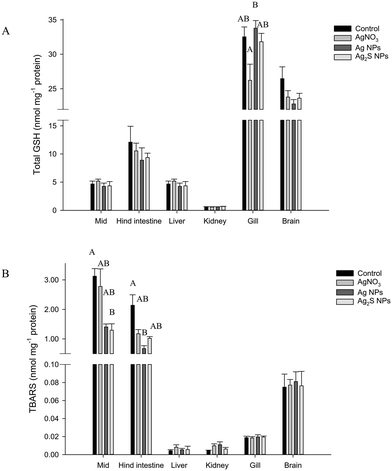
There were no major histological changes to the mid intestine (Fig. 2) or hind intestine (not shown) after 4 weeks of exposure. The mucous epithelium showed normal morphology. There was no evidence of erosion of the tips of the villi and the tissue showed normal columnar epithelial cells without foci of necrosis or reactive hyperplasia. The mucocytes in the epithelial also appeared normal, with no evidence of mucocyte proliferation. The average size of the mucocytes in the mid intestine were 19.2 ± 1.2, 19.8 ± 1.5, 19.8 ± 1.8 and 17.5 ± 0.9 μm in the control, AgNO3, Ag NP and Ag2S NP treatments, and there was no significant difference (one-way ANOVA) between treatments (P = 0.733). Additionally, there was no evidence of pathology to the gills of the Ag treatments or controls, with the absence of oedema in the tips of the secondary lamellae and no evidence of necrosis in the epithelium, or evidence of mucocyte proliferation. The vasculature in the gill appeared normal without aneurisms or evidence of swollen red blood cells. Overall, the normal gill morphology indicated the absence of waterborne exposure to silver.There were no treatment-related differences in the total GSH concentration of the liver, brain, mid intestine, hind intestine or kidney by the end of the exposure (one-way ANOVA or Kruskal–Wallis; Fig. 3). However, there was a small, but statistically significant decrease in total GSH concentration in the gill in the AgNO3 treatment compared to the Ag NP treatment only (P = 0.023), but these effects were not significantly different from the control fish. There were no differences in total GSH between the Ag NP and Ag2S NP treatments at the end of the exposure phase.
There was no change to TBARS concentration of the gill, brain, kidney or liver tissues at the end of the exposure phase (one-way ANOVA or Kruskal–Wallis; Fig. 3). However, some small changes were observed in the intestine (Kruskal–Wallis). The mid intestine of the control fish had a TBARS concentration of 3.1 ± 0.3 nmol TBARS mg−1 protein, whereas the Ag NP and Ag2S NP treatments had 1.4 ± 0.1 and 1.3 ± 0.2 nmol TBARS mg−1 protein. A one-way ANOVA revealed the Ag2S NP treatment was significantly lower compared to the control (P = 0.030) but the Ag NP treatment was not (P = 0.057). Within the hind intestine, there was a trend of decreasing TBARS concentration in all the Ag treatments compared to the controls (the latter, 2.1 ± 0.4 nmol TBARS mg−1 protein), but of these only the Ag NP treatment was significantly reduced compared to the controls (P < 0.001). There were no material-type effects.
In chemico digestibility assay
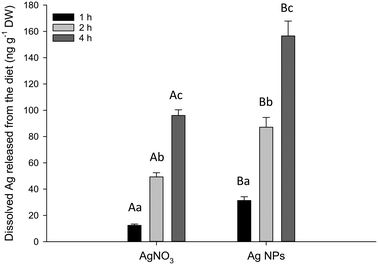
The in chemico digestibility assay was used to identify any potential labile fraction of dissolved silver from the experimental diets at pH 2 to represent the stomach (Fig. 4). There was no detectable dissolution from the control or Ag2S NPs (below the procedural LOD of 2.3 ng g−1). However, there was some release of dissolved Ag from the AgNO3 and Ag NP diets with both time- and treatment-related differences (two-way ANOVA). There was a rapid elevation of dissolved Ag appearing in the external compartment of the test vessel over the 4 hour incubation, with the total Ag (ng) released per g of food approximately doubling between each time interval, in both treatments (Fig. 4). There was significantly more total Ag released from the food pellets containing Ag NPs compared to those with AgNO3 after 1 h (P = 0.040), 2 h (P < 0.001) and 4 h (P < 0.001). There was no detectable dissolution in the intestinal simulations at pH 7.8 (<LOD, data not shown).Discussion
This study has demonstrated that rainbow trout will eat diets contaminated with a nominal 100 mg kg−1 of Ag as AgNO3, Ag NPs or Ag2S NPs without effects on growth or overt toxicity. The ingested silver subsequently caused total silver accumulation (form unknown) in the internal organs including the liver, kidney, brain, and the blood supply. Where accumulation was observed, the internal organs generally showed a gradual increase in accumulation over time, and with either partial or negligible decreases in the organ concentrations during the depuration phase, suggesting only a slow clearance from the body. As a proportion of the body burden, the liver showed the greatest organ accumulation for all treatments, and in keeping with the organ's role as a central compartment in processing metals. While the target organs and pattern of total Ag accumulation was broadly similar for dietary exposure to AgNO3 and Ag NPs, the Ag2S often caused less total Ag accumulation in the internal organs, suggesting the latter nanomaterial was less bioavailable.Dietary exposure and total silver accumulation
It is well known that fish will eat food contaminated with metals (review, Handy et al.6), including Ag,3 usually without mortality. This was also the case in the present study where only a few fish mortalities were observed (in random tanks), and regardless of the form of Ag added to the food, the animals continued to feed and gain body weight (Fig. 1). Dietary Ag exposure was confirmed by the measured total Ag in the food and by carefully monitoring the food intake during the experiment. Incidental waterborne exposure is unlikely because the measured total metal concentrations in the water were at trace levels or below the detection limit and there was no evidence of the gill pathology (e.g., no oedema, no epithelial lifting) that is normally associated with dissolved metal toxicity. There was also no overt pathology in the intestines (Fig. 2), and overall the exposure can be regarded as a sub-lethal event leading to a physiologically relevant pattern of total Ag accumulation in the internal organs.The unexposed control fish showed either trace amounts of total Ag in the organs (<1 μg g−1 dw) or were below detection, in keeping with previous reports of background Ag in trout.3 The dietary exposure to food containing AgNO3 also resulted in the expected pattern of Ag accumulation, with the total Ag accumulating primarily in the intestine and liver compared to unexposed controls, and consistent with the route of exposure. Some total Ag was also detected in the kidney and blood, but not much in the gills of fish fed food containing AgNO3; similar to Galvez et al.3 There appears to be no in vivo reports of dietary exposure to food pellets containing Ag NPs in rainbow trout. The present study showed a pattern of total Ag accumulation (form unknown) in the organs which was very similar to that of the AgNO3 treatment; both in terms of the target organs and the total Ag concentrations achieved in those organs at the end of the exposure phase (Table 1). The distribution of the Ag body burden was also identical between AgNO3 and Ag NP treatments. This suggests the bioaccumulation hazard from dietary AgNO3 and Ag NPs are the same. Recently, Kleiven et al.25 exposed Atlantic salmon to ∼60 mg Ag kg−1 as either 110AgNO3 or as citrate-coated or uncoated 110Ag NPs in a slurry, administered by oral gavage. After two days of exposure, the radioactivity from the AgNO3 and citrate-coated Ag NPs oral treatment, was associated with the liver, but not the gills;25 similar to the findings in the present study with total Ag.
However, Kleiven et al.25 also noticed that radioactivity from the uncoated Ag NPs was transferred less to the liver than the citrate-coated Ag NPs. This suggests the form of the nanomaterial is important to the accumulation pattern. In the present study, the Ag2S treatment generally showed less total Ag accumulation in the internal organs than that of the Ag NPs after four weeks of exposure (Table 1). An in chemico digestibility assay on the same food used in the present study,1 showed that total Ag from the Ag2S-containing pellets was less extractable and therefore would have lower bioavailability in the gut lumen. This is in keeping with the in vivo findings here. However, bioavailability in the gut lumen may not be the only factor that determines Ag accumulation in the internal organs. From a chemistry perspective, Ag2S NPs are regarded as persistent and if they remain inert in biological media, then perhaps they might be less bioaccessible for uptake by the tissues. It is interesting that Kleiven et al.25 found that the radioactivity in the intestine (∼700, 900 and 700 corrected counts per minute per g wet weight tissue, for AgNO3, coated Ag NPs and uncoated Ag NPs, respectively) were similar; and yet less of the radioactivity associated with uncoated Ag NPs was found in the liver.
Depuration and redistribution of the body burden
There are only a few reports of depuration following Ag exposures in fish, and these are for waterborne exposures.26,27 Nonetheless, these studies show, for dissolved silver at least, that the uptake is relatively rapid over hours or a few days and that depuration is slower, over weeks. The depuration phase has an initial exponential decrease in body silver concentrations and then a much slower fraction that persists and is never cleared entirely.26 The dietary exposure to AgNO3, here showed some broad similarity to these earlier waterborne studies with regard to slow clearance. Apart from some clearance from the blood and intestines, there was no appreciable clearance of the body burden after two weeks on normal food (Table 1). However, there was some redistribution of the total Ag towards the liver and kidney, presumably to facilitate eventual excretion and/or inert storage of the total Ag. This was also observed in the Ag NP treatment, and to a lesser extent in the Ag2S treatment (Table 1).Unfortunately, while there have been reports of dietary uptake of metal-containing ENMs in fish (TiO2,15 ZnO,16 CdS particles,17 gold particles,28 quantum dots29); only a few of these studies also measured the carcass with the aim of calculating the body distribution of the total metal. In the present study at the end of the exposure phase, the liver contained 38–44% of the Ag body burden, and regardless of the type of material exposure, this increased to 63–99% after two weeks on normal food (Table 2). This redistribution of the total Ag to the liver, presumably for excretion, has not been previously reported in trout for Ag NPs. The presence of at least some Ag in the gallbladder (Table 1) suggests at least some incidental Ag excretion (form unknown) into the bile from the Ag NP dietary exposure, although the fraction was less than 1% of the body burden (Table 2). At least one study with Ag NPs on marine medaka (Oryzias melastigma) showed retention of Ag from Ag NP dietary exposure,30 but comparisons with freshwater-adapted trout are problematic because of the very different osmoregulatory strategies, renal function, and gut chemistries of marine and freshwater fish.
The kidney may also have a role in depuration and/or storage of Ag (form unknown). The proportion of the body burden in the kidney increased from 4% to 10% in the depuration phase for the Ag NP treatment (Table 2). This might imply some renal excretion of total Ag, or more likely, that the normal macrophage activity in the kidney is resulting in some Ag precipitation in the organ, as is known for Cu NPs.23 The renal perfusion as a proportion of blood flow is also relatively high in freshwater-adapted trout, so the apparent retention by the kidney in the post-exposure phase will inevitably include some total Ag that is in the blood inside the organ. Similar arguments of macrophage activity and blood flow may also apply to the spleen where some total Ag remains (Table 1). Interestingly, van der Zande et al.31 also found that dietary Ag NP exposures in rodents resulted in total Ag accumulation in the internal organs with the most blood flow, such as the liver, spleen, kidney, lung and brain. The rodents also showed some clearance from the blood post-exposure, but with a persistent residual of total Ag in the internal organs, similar to the present study on trout (Tables 1 and 2).
The internal organs of fish fed with Ag2S showed much less total Ag accumulation than either of the other Ag treatments. Consequently, the smaller amounts present were sometimes cleared. For example, at the end of the experiment, the blood and the gills were at the detection limit for total Ag (Table 1); but otherwise the pattern for the depuration phase was similar to the Ag NPs. However, on a proportion of body burden basis, the carcass from the Ag2S treatment cleared all the detectable total Ag compared to the other treatments by week 6 (Table 2).
Growth, gut health and sub-lethal effects
One concern for dietary exposure to metals is the potential for adverse effects on growth, nutritional performance, or on the integrity of the gut epithelium. In the present study, there were no effects of any of the Ag-containing treatments on cumulative food intake or growth (Fig. 1). The intestines showed some reductions in TBARS in the animals fed with nanomaterials, but without total glutathione depletion in any treatment (Fig. 3), and the intestinal morphology was normal (Fig. 2). Together, this suggests the gut remained healthy, despite the potential for some dissolved silver release in the acidic conditions of the stomach (Fig. 4). There are only a few in vivo reports of nutritional performance in rainbow trout few diets containing added silver salts. Galvez and Wood4 fed trout diets containing up to 3000 mg kg−1 of Ag as Ag2S for 58 days with no significant differences in food intake, specific growth rate or food conversion efficiency; despite clear Ag accumulation in the intestines. Galvez et al.3 made similar observations on growth and food intake with trout fed 3.1 mg kg−1 of Ag over 126 days, where the Ag has been biologically incorporated into trout meal used to make the food pellets.The absence of intestinal pathology with dietary AgNO3 (Fig. 2) is perhaps not surprising. The gut was well-defended with intact mucous cells (Fig. 2), and the tissue depending on the region of the gut in trout, typically contains between 100–500 μg g−1 wet weight of metallothionein;32 which together might readily chelate the maximum total Ag of around 140 μg g−1 found in the hind intestine (Table 1). There appears to be no reports of in vivo intestinal morphology in trout fed dietary Ag NPs, but in zebrafish fed 500 mg kg−1 of Ag NPs for 14 days there was no loss of integrity of the gut epithelium or any damage to the microvilli on the apical surface of the gut cells.33 Rats receiving a daily administration of 3.6 mg kg−1 by gavage of cubic or spherical Ag NPs (20 mL kg−1 body weight) for 14 days also showed no evidence of histological disturbance to the stomach, small intestine, cecum or colon.34
Exposure to dissolved silver via the water is known to interfere with branchial sodium homeostasis in trout.35 However, dietary silver generally does not. Galvez et al.3 found no effects of dietary Ag on Na+ influx or plasma Na+ in trout. Similarly for the AgNO3 diet in the present study, there were no effects on the total glutathione or TBARS in the gill (Fig. 3), indicating negligible oxidative stress to this osmoregulatory organ. There were no effects on plasma Na+, and only small changes in plasma K+ within the physiological range, compared to controls (Table S1†). The major electrolytes in the internal organs was also unaffected (Table S2†). The same observations were made for dietary exposure to Ag NPs or Ag2S NPs (Tables S1 and S2†). There were some statistically significant disturbances to Cu concentrations in some organs (Table S2†), and this was observed previously in dietary studies with TiO2 NPs in trout,15 although the biological importance of small changes in tissue Cu is unclear. In the present study, there was no evidence of oxidative stress in the internal organs (Fig. 3).
Conclusions and perspective on environmental hazard assessment
In conclusion, the present study has demonstrated dietary accumulation of total Ag in trout arising from exposures to food containing AgNO3, Ag NPs or Ag2S NPs. The data is interpreted as sub-lethal, with physiologically relevant uptake and without pathology to the gut or biochemical disturbances to the internal organs. A key concern for environmental regulation is whether ENMs present a different hazard to their nearest equivalent metal salt. The present study showed the dietary bioaccumulation of AgNO3 and Ag NPs to be equal, with total silver from both of these materials (form unknown in the tissue) being more bioavailable than total Ag arising from the Ag2S NP treatment. The bioaccumulation potential ranking would therefore be AgNO3 = Ag NPs > Ag2S NPs. However, there is also a desire to reduce the use of animals in the bioaccumulation testing strategy.1 Recently, our laboratory used an alternative ex vivo gut sac technique for assessing the total Ag accumulation by the gut in 4 hour incubations using the same ENMs as the present study.20 The gut sac approach is intended as a screening tool in a tiered approach to bioaccumulation testing,1 and correctly identified a bioaccumulation concern for both AgNO3 and Ag-containing ENMs in the gut tissue. The ex vivo gut sac results gave a slightly different ranking of AgNO3 > Ag NPS = Ag2S NPs.20 Regardless, both the in vivo and ex vivo findings on these materials suggest the existing risk assessment for dissolved silver would encompass the bioaccumulation potential risk of the nano forms (i.e., no additional risk from nano). In both studies, the most likely of the nano forms to be released into the environment, the Ag2S NPs, was identified as less hazardous than AgNO3. However, further work is needed to understand how the food matrix can effect dietary bioavailability of ENMs and to determine if ENMs can become ‘biologically incorporated’ into the tissues in a similar fashion to dissolved metals. The human health risk from the total silver accumulated in the carcass (i.e., mostly the edible flesh) also needs to be considered, and work is underway to validate a detection method for nano forms and particle size distributions in the internal organs of trout.Conflicts of interest
There are no conflicts of interest.Acknowledgements
This work was supported by the EU H2020 NanoFase project, grant agreement no. 646002. The authors would like to thank Andrew Atfield and Dr Will Vevers for technical support, and Dr Richard Maunder, Dr Audrey Barranger, and Charlotte Crowther for assistance on dissection days. Additionally, we thank Dr Rob Clough and Dr Andrew Fisher for help with ICP-MS.References
- R. D. Handy, J. Ahtiainen, J. M. Navas, G. Goss, E. A. Bleeker and F. von der Kammer, Proposal for a tiered dietary bioaccumulation testing strategy for engineered nanomaterials using fish, Environ. Sci.: Nano, 2018, 5, 2030–2046 RSC.
- H. T. Ratte, Bioaccumulation and toxicity of silver compounds: a review, Environ. Toxicol. Chem., 1999, 18, 89–108 CrossRef CAS.
- F. Galvez, C. Hogstrand, J. C. McGeer and C. M. Wood, The physiological effects of a biologically incorporated silver diet on rainbow trout (Oncorhynchus mykiss), Aquat. Toxicol., 2001, 55, 95–112 CrossRef CAS PubMed.
- F. Galvez and C. M. Wood, Physiological effects of dietary silver sulfide exposure in rainbow trout, Environ. Toxicol. Chem., 1999, 18, 84–88 CrossRef CAS.
- S. J. Clearwater, A. M. Farag and J. S. Meyer, Bioavailability and toxicity of dietborne copper and zinc to fish, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2002, 132, 269–313 CrossRef.
- R. D. Handy, J. C. McGeer, H. E. Allen, P. E. Drevnick, J. W. Gorsuch, A. S. Green, A.-K. Lundebye-Haldorsen, S. E. Hook, D. R. Mount and W. A. Stubblefield, in Toxicity of Dietborne Metals to Aquatic Organisms, ed. W. J. A. J. S. Meyer, K. V. Brix, S. N. Luoma, D. R. Mount, W. A. Stubblefield and C. M. Wood, SETAC Press Pensacola, USA, 2005, pp. 59–112 Search PubMed.
- S. J. Klaine, P. J. J. Alvarez, G. E. Batley, T. F. Fernandes, R. D. Handy, D. Y. Lyon, S. Mahendra, M. J. McLaughlin and J. R. Lead, Nanomaterials in the environment: Behavior, fate, bioavailability, and effects, Environ. Toxicol. Chem., 2008, 27, 1825–1851 CrossRef CAS PubMed.
- H. Selck, R. D. Handy, T. F. Fernandes, S. J. Klaine and E. J. Petersen, Nanomaterials in the aquatic environment: A European Union–United States perspective on the status of ecotoxicity testing, research priorities, and challenges ahead, Environ. Toxicol. Chem., 2016, 35, 1055–1067 CrossRef CAS PubMed.
- R. K. Cross, C. Tyler and T. S. Galloway, Transformations that affect fate, form and bioavailability of inorganic nanoparticles in aquatic sediments, Environ. Chem., 2015, 12, 627–642 CrossRef CAS.
- C. Levard, E. M. Hotze, G. V. Lowry and G. E. Brown Jr, Environmental transformations of silver nanoparticles: impact on stability and toxicity, Environ. Sci. Technol., 2012, 46, 6900–6914 CrossRef CAS PubMed.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Transformations of nanomaterials in the environment, Environ. Sci. Technol., 2012, 46, 6893–6899 CrossRef CAS PubMed.
- J. R. Lead, G. E. Batley, P. J. Alvarez, M. N. Croteau, R. D. Handy, M. J. McLaughlin, J. D. Judy and K. Schirmer, Nanomaterials in the environment: behavior, fate, bioavailability, and effects — An updated review, Environ. Toxicol. Chem., 2018, 37, 2029–2063 CrossRef CAS PubMed.
- R. Kaegi, A. Voegelin, C. Ort, B. Sinnet, B. Thalmann, J. Krismer, H. Hagendorfer, M. Elumelu and E. Mueller, Fate and transformation of silver nanoparticles in urban wastewater systems, Water Res., 2013, 47, 3866–3877 CrossRef CAS PubMed.
- J. H. Bisesi Jr, J. Merten, K. Liu, A. N. Parks, A. N. Afrooz, J. B. Glenn, S. J. Klaine, A. S. Kane, N. B. Saleh and P. L. Ferguson, Tracking and quantification of single-walled carbon nanotubes in fish using near infrared fluorescence, Environ. Sci. Technol., 2014, 48, 1973–1983 CrossRef PubMed.
- C. S. Ramsden, T. J. Smith, B. J. Shaw and R. D. Handy, Dietary exposure to titanium dioxide nanoparticles in rainbow trout, (Oncorhynchus mykiss): no effect on growth, but subtle biochemical disturbances in the brain, Ecotoxicology, 2009, 18, 939–951 CrossRef CAS PubMed.
- M. Connolly, M. Fernandez, E. Conde, F. Torrent, J. M. Navas and M. L. Fernandez-Cruz, Tissue distribution of zinc and subtle oxidative stress effects after dietary administration of ZnO nanoparticles to rainbow trout, Sci. Total Environ., 2016, 551, 334–343 CrossRef PubMed.
- C. Ladhar, B. Geffroy, S. Cambier, M. Treguer-Delapierre, E. Durand, D. Brèthes and J.-P. Bourdineaud, Impact of dietary cadmium sulphide nanoparticles on Danio rerio zebrafish at very low contamination pressure, Nanotoxicology, 2014, 8, 676–685 CrossRef CAS PubMed.
- M. F. El Basuini, A. M. El-Hais, M. A. Dawood, A. E.-S. Abou-Zeid, S. Z. EL-Damrawy, M. M. E.-S. Khalafalla, S. Koshio, M. Ishikawa and S. Dossou, Effect of different levels of dietary copper nanoparticles and copper sulfate on growth performance, blood biochemical profiles, antioxidant status and immune response of red sea bream (Pagrus major), Aquaculture, 2016, 455, 32–40 CrossRef CAS.
- M. Baccaro, A. K. Undas, J. de Vriendt, J. van den Berg, R. Peters and N. W. van den Brink, Ageing, dissolution and biogenic formation of nanoparticles: how do these factors affect the uptake kinetics of silver nanoparticles in earthworms?, Environ. Sci.: Nano, 2018, 5, 1107–1116 RSC.
- N. J. Clark, D. Boyle and R. D. Handy, An assessment of the dietary bioavailability of silver nanomaterials in rainbow trout using an ex vivo gut sac technique, Environ. Sci.: Nano, 2019, 6, 646–660 RSC.
- T. W. K. Fraser, H. C. Reinardy, B. J. Shaw, T. B. Henry and R. D. Handy, Dietary toxicity of single-walled carbon nanotubes and fullerenes (C60) in rainbow trout (Oncorhynchus mykiss), Nanotoxicology, 2011, 5, 98–108 CrossRef CAS PubMed.
- B. J. Shaw, G. Al-Bairuty and R. D. Handy, Effects of waterborne copper nanoparticles and copper sulphate on rainbow trout, (Oncorhynchus mykiss): Physiology and accumulation, Aquat. Toxicol., 2012, 116, 90–101 CrossRef PubMed.
- G. A. Al-Bairuty, B. J. Shaw, R. D. Handy and T. B. Henry, Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss), Aquat. Toxicol., 2013, 126, 104–115 CrossRef CAS PubMed.
- C. J. Smith, B. J. Shaw and R. D. Handy, Toxicity of single walled carbon nanotubes to rainbow trout, (Oncorhynchus mykiss): respiratory toxicity, organ pathologies, and other physiological effects, Aquat. Toxicol., 2007, 82, 94–109 CrossRef CAS PubMed.
- M. Kleiven, B. O. Rosseland, H. C. Teien, E. J. Joner and D. Helen Oughton, Route of exposure has a major impact on uptake of silver nanoparticles in Atlantic salmon (Salmo salar), Environ. Toxicol. Chem., 2018, 37, 2895–2903 CrossRef CAS PubMed.
- C. M. Wood, M. Grosell, C. Hogstrand and H. Hansen, Kinetics of radiolabelled silver uptake and depuration in the gills of rainbow trout (Oncorhynchus mykiss) and European eel (Anguilla anguilla): the influence of silver speciation, Aquat. Toxicol., 2002, 56, 197–213 CrossRef CAS PubMed.
- C. Hogstrand, M. Grosell, C. M. Wood and H. Hansen, Internal redistribution of radiolabelled silver among tissues of rainbow trout (Oncorhynchus mykiss) and European eel (Anguilla anguilla): the influence of silver speciation, Aquat. Toxicol., 2003, 63, 139–157 CrossRef CAS PubMed.
- B. Geffroy, C. Ladhar, S. Cambier, M. Treguer-Delapierre, D. Brèthes and J.-P. Bourdineaud, Impact of dietary gold nanoparticles in zebrafish at very low contamination pressure: the role of size, concentration and exposure time, Nanotoxicology, 2012, 6, 144–160 CrossRef CAS PubMed.
- N. A. Lewinski, H. Zhu, C. R. Ouyang, G. P. Conner, D. S. Wagner, V. L. Colvin and R. A. Drezek, Trophic transfer of amphiphilic polymer coated CdSe/ZnS quantum dots to Danio rerio, Nanoscale, 2011, 3, 3080–3083 RSC.
- J. Wang and W.-X. Wang, Low bioavailability of silver nanoparticles presents trophic toxicity to marine medaka (Oryzias melastigma), Environ. Sci. Technol., 2014, 48, 8152–8161 CrossRef CAS PubMed.
- M. van der Zande, R. J. Vandebriel, E. Van Doren, E. Kramer, Z. Herrera Rivera, C. S. Serrano-Rojero, E. R. Gremmer, J. Mast, R. J. Peters and P. C. Hollman, Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure, ACS Nano, 2012, 6, 7427–7442 CrossRef CAS PubMed.
- M. Chowdhury, B. Baldisserotto and C. Wood, Tissue-specific cadmium and metallothionein levels in rainbow trout chronically acclimated to waterborne or dietary cadmium, Arch. Environ. Contam. Toxicol., 2005, 48, 381–390 CrossRef CAS PubMed.
- D. L. Merrifield, B. J. Shaw, G. M. Harper, I. P. Saoud, S. J. Davies, R. D. Handy and T. B. Henry, Ingestion of metal-nanoparticle contaminated food disrupts endogenous microbiota in zebrafish (Danio rerio), Environ. Pollut., 2013, 174, 157–163 CrossRef CAS PubMed.
- A. B. Javurek, D. Suresh, W. G. Spollen, M. L. Hart, S. A. Hansen, M. R. Ellersieck, N. J. Bivens, S. A. Givan, A. Upendran and R. Kannan, Gut dysbiosis and neurobehavioral alterations in rats exposed to silver nanoparticles, Sci. Rep., 2017, 7, 2822 CrossRef PubMed.
- M. Grosell, C. Nielsen and A. Bianchini, Sodium turnover rate determines sensitivity to acute copper and silver exposure in freshwater animals, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2002, 133, 287–303 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9en00261h |
| This journal is © The Royal Society of Chemistry 2019 |