Metabolomics reveals that engineered nanomaterial exposure in soil alters both soil rhizosphere metabolite profiles and maize metabolic pathways†
Lijuan
Zhao
*a,
Huiling
Zhang
 a,
Jason C.
White
a,
Jason C.
White
 b,
Xiaoqiang
Chen
a,
Hongbo
Li
a,
Xiaolei
Qu
a and
Rong
Ji
*a
b,
Xiaoqiang
Chen
a,
Hongbo
Li
a,
Xiaolei
Qu
a and
Rong
Ji
*a
aState Key Laboratory of Pollution Control and Resource Reuse, School of Environment, Nanjing University, Nanjing 210023, China. E-mail: ljzhao@nju.edu.cn; ji@nju.edu.cn; Fax: +86 025 8968 0581; Tel: +86 025 8968 0581
bDepartment of Analytical Chemistry, The Connecticut Agricultural Experiment Station (CAES), New Haven, Connecticut 06504, USA
First published on 25th April 2019
Abstract
Accurate risk assessment of engineered nanomaterials (ENMs) in the environment is important for sustainable development and application of nanotechnology. Soil metabolomics, which reflects the integrated response of both plant and microbial communities to ENM exposure, has not been used extensively. Moreover, since microbe- and plant-released metabolites contribute to the formation and accumulation of soil organic carbon (SOC), soil metabolite profile alteration from impacted plant and microbial activity may change SOC pool enrichment. Here, maize plants were grown in soil amended with SiO2, TiO2, or Fe3O4 ENMs (100 mg kg−1 soil) for four weeks. Plant and soil metabolomics were then used to investigate the global metabolic response of both the plant and soil to ENM exposure. None of the tested ENMs showed negative impacts on plant growth. However, metabolomics analysis revealed that all ENM treatments altered the leaf, root and soil metabolite profiles in an ENM-dependent manner. Fe3O4 and TiO2 ENM exposure induced stronger metabolic reprogramming in leaves, roots and soil compared to SiO2 ENMs. Interestingly, leaf tissues, which is not the organ directly exposed to ENMs, showed significant amino acid pool alteration upon exposure to ENMs. In soil, levoglucosan, linolenic acid, 4-hydroxycinnamic acid and allo-inositol were significantly increased in response to ENMs. Alteration of the soil metabolite profile indicates that ENMs changed the SOC pool. Integration of leaf, root and soil metabolomics enables a thorough characterization of plant metabolism and soil chemistry that can be a powerful tool for ENM risk assessment.
Environmental significanceAccurate risk assessment of engineered nanomaterials (ENMs) in the environment is important for sustainable development and application of nanotechnology. However, previous studies have focused on the response of either plant or soil microbial communities separately. Soil metabolomics, which reflects the integrated response of both plant and microbial communities to ENM exposure, has not been used extensively. Here, maize plants were grown in soil amended with SiO2, TiO2, or Fe3O4 ENMs (100 mg kg−1 soil) for four weeks. Metabolomics analysis revealed that all ENM treatments altered the leaf, root and soil metabolite profiles in an ENM-dependent manner. Integration of leaf, root and soil metabolomics enables a thorough characterization of plant metabolism and soil chemistry that can be a powerful tool for ENM risk assessment. |
Introduction
Although engineered nanomaterials (ENMs) have tremendous potential for beneficial impacts in a wide range of sectors, the risk associated with their uses should still be thoroughly evaluated.1 Agricultural soil will be a primary sink for many ENMs through a number of input pathways. A robust study on the safety assessment of ENMs to crop plants has been developed, including endpoints from phenotypic, metabolomic, proteomic and transcriptomic levels using systems biology approaches. Meanwhile, the impact of ENM exposure on microbial communities in soil or sludge has also been studied using high-throughput sequencing platforms.2–6 Importantly, soil is the place where plant roots and important rhizosphere microbes co-exist and engage in symbiotic relationships critical to the ecosystem function. However, few studies have sought to integrate plant and soil response to ENM exposure.Plant and soil microbial communities are connected by a number of pathways, including surfaces and by root exudation.7 Plants transport 5–21% of their photosynthetically fixed carbon to the roots for exudation as soluble sugars, amino acids, and carboxylic acids, as well as a diverse set of secondary metabolites. These organic constituents enter the rhizosphere, and are used by the microbes as carbon and energy sources, as well as signaling molecules.8,9 Soil is the critical location of plant root and soil microbe interaction. Plant and soil microbial metabolic activities ultimately govern the soil metabolic profile. Quantifying low molecular weight metabolites in soil can provide an integrated assessment of both plant and soil simultaneously. On the other hand, soil organic matter is the largest terrestrial carbon pool,10,11 and both microbes and plants contribute to the formation and accumulation of this SOC.12 ENMs can indirectly impact the SOC pool by modulating the metabolic activities of both microbes and plants. Evaluating the soil metabolite composition and the amounts of individual metabolites will reveal how the SOC pool is impacted by ENM exposure.
Metabolomics is a powerful and high-throughput tool to capture and analyze a snapshot of the metabolic status of a plant at a given point in time, often in an untargeted manner.13 Similarly, metabolomics can be used to quantify soil metabolites (soil metabolomics) and provide a similar profile of soil chemical composition status under various conditions, including stress from contaminant exposure. Swenson et al. proposed “soil metabolomics” methods for analysis of the soil organic matter pool.14 Soil metabolites largely consist of small molecules of plant and microbial origin resulting from lysed cells and released metabolites.15 Jones et al.16 proposed the concept of “community metabolomics”, which is the application of metabolomics techniques to study the entire community of a soil sample. The application of soil metabolomics as part of a comprehensive safety assessment of ENMs has been less studied.
Fe3O4 magnetic nanoparticles (NPs) have catalytic and adsorbent activity, as well as antimicrobial properties, and thus have wide use in biomedical applications, water treatment and soil remediation. Nanoparticle TiO2 also has wide use in personal care products, antimicrobial products and as a catalyst.17 In addition, both TiO2 and SiO2 have shown potential as agricultural amendments for pest and fungal control.18 Thus, application of these ENMs in environmental remediation and nano-enabled agriculture will lead to their accumulation in soil, making risk assessment of the use of these materials critical to their safe and sustainable application.
Maize (Zea mays) is a globally important crop with an estimated global production of 1.05 billion tonnes in 2017.19 Therefore, we chose maize as a model crop to evaluate the fate and effects of ENMs. Here, maize plants were cultivated in soil amended with different ENMs (Fe3O4, SiO2 and TiO2) at a dose of 100 mg kg−1, which are likely to produce changes in the soil microbial community and the dose was also within the realm of environmental relevance. Upon exposure to ENMs, the leaf, root and soil metabolite profile was evaluated using gas chromatography-mass spectrometry (GC-MS). This molecular approach provides a new level of insight into ENM effects and will significantly give information on risk assessment for ENMs and can be used for other contaminant systems.
Materials and methods
Nanoparticles and plants
SiO2 and TiO2 NPs were purchased from Pantian Nano Material Inc. (99.9%, Shanghai, China) and Fe3O4 NPs were purchased from Xiangtian Nano Material Inc. (99.9%, Shanghai, China). The transmission electron microscopy (TEM) images of the NPs are shown in Fig. S1.† The original size for SiO2, TiO2 and Fe3O4 NPs are approximately 20, 5–10, and 30 nm, respectively (Table S1†). To determine the particle hydrodynamic size and zeta potential (Malvern, Nano Series ZS90) in nanopure water, 100 mg L−1 ENM stock solutions were prepared and bath-sonicated (KH-140100DB, Hechuang Ultrasonic, Jiangsu, China) at 45 kHz for 30 min before determination. The average hydrodynamic sizes for SiO2, TiO2 and Fe3O4 were 876 ± 41, 592 ± 7, and 1230 ± 56 nm, respectively, with the corresponding zeta potentials of 19.5 ± 0.37, −21.4 ± 0.64 and 11.6 ± 0.64 mV, respectively (Table S1†). Maize (Zea mays) seeds were obtained from Hezhiyuan Seed Corporation (Shandong, China).Exposure assay
The agricultural soil was collected from agricultural experimental stations of Chinese Academy of Sciences at Hailun (site N, 126° 38′E and 47° 26′N), from the top 20 cm. The stock solutions of 1000 mg L−1 of SiO2, TiO2 and Fe3O4 were prepared in nanopure water. Before application to soil, the suspension was bath-sonicated at 45 kHz for 30 min in cool water until a stable dispersion was obtained. The final dose for all ENMs was 100 mg kg−1 soil. Plastic containers (9 cm × 9 cm × 7 cm) were then filled with 100 g growth media (40 g agricultural soil and 60 g of potting soil). The potting soil (0.68% N, 0.27% P2O5, and 0.36% K2O, pH 4.28) (Miracle-Gro, Beijing, China) was added to ensure an adequate nutrient supply during cultivation, specifically to avoid nutrient-stress that might skew plant response. In total, there were four treatments: control (no ENMs), 100 mg kg−1 SiO2, 100 mg kg−1 TiO2 and 100 mg kg−1 Fe3O4. Four replicate plants (two plants per pot) were grown for each treatment. The plants were cultivated in a greenhouse for 28 days at 25 °C during the day and 20 °C at night. The daily light integral was 180 μmol m−2 s−1. During the growth period, the plants were watered as needed and no additional fertilizers were applied.Biomass and chlorophyll content analysis
At harvest, maize plants were thoroughly rinsed with tap water for 5 min followed by deionized water 3 times. The fresh biomass of root, stem and leaf tissues was determined before oven-drying (60 °C for 72 h). Photosynthetic pigment analysis was performed to determine the levels of chlorophyll a and b and total carotenoids. The pigments were extracted following the protocol of Sestak et al.20 Briefly, 0.01 g of maize leaves were mixed with 5 mL of 80% methanol for 12 h, and then the mixture was centrifuged for 10 min at 3000 rpm. A microplate spectrophotometer (Biotek Synergy H1, America) was used to measure the absorbance of chlorophyll a and b and carotenoids in the methanolic extracts at 663, 645, and 470 nm, respectively.ICP-MS analysis for element content
Tissues for elemental analysis were dried at 60 °C for 72 h. A sample of approximately 0.02 g of dried tissue was microwave-digested (Milestone, Ethos Up, Germany) in a mixture of 8 mL of H2O2 and 2 mL of HNO3 (4/1 v/v) at 160 °C for 40 min. In terms of Ti, 0.1 g of dried tissues were digested in a mixture of 1 mL of H2O2, 2 mL of HNO3 and 5 mL of H2SO4 at 180 °C for 15 min according to the sample digestion method described by Larue et al.21 The resulting digestion solution was diluted to a final volume of 50 mL prior to analysis. The content of Fe, Si, Ti and other macro- and micro-nutrients (K, Ca, Mg, Cu and Mn) were quantified by inductively coupled plasma-optical emission spectroscopy (ICP-OES) (Optima 8300, Perkin Elmer, U.S.A) or ICP-mass spectrometry (MS) (NexION-300, PerkinElmer).Metabolite analysis in maize tissues and soil
The freeze-dried leaf, root and soil samples were subjected to GC-MS based metabolomics analysis. Details on metabolite extraction, GC-MS analysis, and multivariate analysis are described below.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) by sonicating at 60 HZ for 2 min. Additional details regarding the extraction of metabolites from tissues and soil are described in the ESI.†
1) by sonicating at 60 HZ for 2 min. Additional details regarding the extraction of metabolites from tissues and soil are described in the ESI.†
Multivariate statistical analysis
A supervised partial least-squares discriminant analysis (PLS-DA) clustering method was conducted on the GC-MS data via online resources (http://www.metaboanalyst.ca/).22 Before PLS-DA analysis, data normalization was performed (normalization by sum) for general-purpose adjustment for the difference among samples, and data transformation (log transformation) was conducted to make individual features more comparable. Variable importance in projection (VIP) is the weighted sum of the squares of the PLS-DA analysis, and indicates the importance of a variable to the entire model.23 A variable with a VIP greater than 1 is regarded as responsible for separation, and is defined as a discriminating metabolite in this study.24 Biological pathway analysis was performed based on GC-MS data using MetaboAnalyst 4.0.25 The impact value threshold calculated for pathway identification was set at 0.1.24Univariate statistical analysis
For the assay of biomass, MDA, photosynthetic pigments and metal content, significant differences between treatment and control means were evaluated using an independent two sample t-test. Reference to a significant difference between treatment means is based on a probability of p < 0.05, unless otherwise stated. Data are presented as mean ± standard errors (n = 4).Results and discussion
Chlorophyll content and biomass
Exposure to ENMs did not result in signs of overt toxicity or stress (Fig. 1A). Similarly, the levels of photosynthetic pigments (chlorophyll a, b and carotenoid) were unchanged upon SiO2 and TiO2 ENM exposure. However, Fe3O4 ENMs resulted in a significant (p < 0.05) increase (22.7%) in chlorophyll b content (Fig. 1B). This is consistent with previous studies demonstrating that superparamagnetic iron oxide increased the chlorophyll content of soybean.26 In addition, Fe3O4 ENMs significantly (p < 0.01) increased leaf biomass by 15% compared to the control; root and stem biomass were not significantly impacted. However, total fresh biomass was significantly (p < 0.05) increased by Fe3O4 NPs as well (Fig. 1C). Similarly, Rui et al. reported that nano-Fe2O3 enhanced peanut biomass and chlorophyll content at 1000 mg kg−1.27 The mechanism for Fe3O4 enhancing chlorophyll b is still unknown. In contrast, SiO2 and TiO2 NPs did not significantly impact maize biomass, which is different from previous reports. Zahra et al.28 reported that 50 mg kg−1 TiO2 significantly increased shoot dry weight and length of Lactuca sativa. Rafique et al.29 also observed a positive impact of TiO2 NPs (up to 100 mg kg−1) on root length and biomass of wheat seedlings. It is assumed that the observed phytotoxicity of ENMs depends on the plant species, particle size, and surface charge.Malondialdehyde (MDA) content, which is an indicator of membrane lipid peroxidation, was determined to evaluate the influence of ENM exposure on cell membrane integrity. The results show that none of the ENMs increased the MDA levels in either the root or leaf tissues of the maize plant (Fig. 1D), indicating that no lipid peroxidation had been induced. Conversely, TiO2 exposure significantly decreased the MDA content (p < 0.05) in maize leaves. This may indicate that TiO2 ENMs have a protective role in alleviating plant oxidative stress. A decreased MDA level in Coriandrum sativum was also observed in the presence of 400 mg kg−1 ZnO NPs by Pullagurala et al.30
Distribution of Si, Ti, Fe and nutrient elements in the plant and soil
SiO2 ENMs are known to be relatively stable in the rhizosphere, whereas TiO2 and Fe3O4 are more likely to undergo dissolution, releasing Ti (Ti4+) and Fe ions (Fe3+ and Fe2+) into the soil solution and rhizosphere.31 We found that Si, Fe and Ti contents in maize tissues (leaf and root) and their water soluble content (bioavailable) in soil were unchanged compared to controls (Table 1), indicating no uptake and translocation of Si/SiO2, Ti/TiO2 and Fe/Fe3O4 NPs in the maize plant. These results also indicate that the release of Fe ions from Fe3O4 NPs is negligible, although the soil pH significantly decreased from 5.35 to 5.13 (p < 0.05) in the presence of Fe3O4 NPs during four weeks of cultivation. This result is consistent with the study of Antisari et al.32 who reported that the solubility of Fe3O4 NPs in soil is low.| K | Ca | Mg | Fe | Ti | Cu | Mn | Si | |
|---|---|---|---|---|---|---|---|---|
| * indicates the statistical difference compared to the control based on the t-test at P ≤ 0.05, ** P ≤ 0.001. | ||||||||
| Leaf | ||||||||
| Control | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 191 ± 1451 191 ± 1451 |
2355 ± 392 | 2482 ± 218 | 127 ± 67 | 15 ± 3 | 66 ± 6 | 11 ± 1 | 711 ± 82 |
| SiO2 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 848 ± 2525 848 ± 2525 |
2094 ± 176 | 2408 ± 242 | 106 ± 27 | 12 ± 3 | 61 ± 6 | 14 ± 7 | 609 ± 40 |
| TiO2 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 109 ± 1690 109 ± 1690 |
2204 ± 355 | 2524 ± 320 | 99 ± 19 | 11 ± 0 | 67 ± 9 | 10 ± 1 | 704 ± 49 |
| Fe3O4 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 954 ± 2576 954 ± 2576 |
2669 ± 186 | 2904 ± 99* | 90 ± 16 | 13 ± 2 | 74 ± 2 | 12 ± 1** | 989 ± 227 |
| Root | ||||||||
| Control | 5288 ± 403 | 3856 ± 318 | 1757 ± 152 | 1441 ± 244 | 103 ± 26 | 22 ± 9 | 86 ± 19 | 2772 ± 489 |
| SiO2 | 5363 ± 677 | 4026 ± 303 | 1860 ± 155 | 1417 ± 252 | 113 ± 39 | 15 ± 1 | 113 ± 26 | 2738 ± 534 |
| TiO2 | 4659 ± 699 | 3670 ± 227 | 1751 ± 159 | 1302 ± 357 | 102 ± 17 | 50 ± 56 | 101 ± 14 | 2898 ± 730 |
| Fe3O4 | 5501 ± 487 | 4275 ± 408 | 1807 ± 166 | 1151 ± 332 | 77 ± 24 | 18 ± 1 | 98 ± 19 | 2234 ± 767 |
| Soil | ||||||||
| Control | 7 ± 1.2 | 108 ± 63 | 31 ± 16 | 8 ± 2 | 0.25 ± 0.07 | 0.064 ± 0.015 | 0.47 ± 0.28 | 4.74 ± 0.13 |
| SiO2 | 7 ± 3.1 | 62 ± 8 | 20 ± 3 | 14 ± 6 | 0.30 ± 0.08 | 0.034 ± 0.005* | 0.17 ± 0.03 | 4.93 ± 1.24 |
| TiO2 | 5 ± 0.8* | 87 ± 26 | 25 ± 7 | 7 ± 2 | 0.24 ± 0.07 | 0.027 ± 0.007** | 0.17 ± 0.07 | 3.39 ± 1.02 |
| Fe3O4 | 7 ± 0.7 | 110 ± 38 | 31 ± 10 | 10 ± 5 | 0.14 ± 0.07 | 0.023 ± 0.010** | 0.25 ± 0.07 | 4.47 ± 2.36 |
Interestingly, exposure to ENMs (SiO2, TiO2, and Fe3O4) significantly (p < 0.05) decreased soil water soluble Cu by 47–64% compared to the control (Table 1). It is possible that ENMs act as an adsorbent or chelator for copper ions and reduced the soil soluble amount of the element. An alternative explanation is that these NMs triggered some metabolite release to soil as root exudates, which chelate with Cu ions and lowered the bioavailability of Cu. For example, organic acid and nicotianamine have been found to be able to bind with metal ions. In root tissues, ENM exposure had no impact on the level of mineral nutrients. However, Mg and Mn content in the leaves under Fe3O4 treatment was significantly (p < 0.05) increased by 18% and 5%, respectively, compared with the control. Magnesium is the central atom of the chlorophyll molecule and many key chloroplast enzymes are strongly affected by the presence of magnesium ions.33 Manganese, as an activator for a number of enzyme reactions in plants, activates several important metabolic reactions and plays an important role in photosynthesis. Therefore, increases in leaf Mg and Mn likely contributed directly to the increased chlorophyll content and leaf biomass. However, the underlying mechanism for Fe3O4 increased Mg and Mn contents in leaves is still under investigation.
Impact of ENMs on leaf metabolome
Using GC-MS, 287 metabolites were identified and semi-quantified in maize leaves. To visualize general grouping information as a function of treatment, PLS-DA analysis was performed. The loading plot (Fig. 2A) reveals a clear separation in the first principle component for the composition of leaf metabolites based on exposure to different ENMs compared to the control, explaining 14.7% of the variation in the data. These results clearly indicate that ENM exposure induced alteration in the maize leaf metabolite profile, which is somewhat unexpected given the lack of direct exposure to this tissue and the lack of observed phenotypic changes. Bezemer and Dam showed that belowground pathogenic microorganisms can also induce defense responses of aboveground tissues and vice versa.34 It is clear that below ground tissues transport and deliver the long-distance signal to the upper tissues, resulting in metabolic alterations in leaves. We also note that the metabolic changes are ENM-dependent, with Fe3O4 inducing the most noticeable metabolic changes, followed by TiO2, and SiO2 (Fig. 2A).In order to discern potential patterns underlying the tested ENMs, a univariate analysis (one-way ANOVA) was run and revealed that 49 metabolites (Table S2†) were found to be significantly changed by exposure. Interestingly, all ENMs resulted in some common generalized metabolic changes, specifically, the triggering of strong amino acid perturbations in the maize leaves. This is particularly interesting given that the ENMs have substantially different physiochemical characteristics. A number of amino acids were either up-regulated (glutamic acid, isoleucine, serine, valine, tyrosine, phenylalanine, and threonine) or down-regulated (glutamine, aspartic acid, glycine, and proline) upon exposure to ENMs (Fig. 3). The reason why ENMs induced pronounced amino acid pool changes is still unknown, however, amino acids play a central role in a wide variety of plant physiological processes, including providing the building blocks of proteins and osmolytes, regulating ion transport, participating in heavy metal detoxification and affecting the synthesis and activity of many critical cellular enzymes.35,36 Tyrosine and phenylalanine are precursor compounds for a variety of secondary metabolites such as phenylpropanoids, alkaloids and glucosinolates,36 the up-regulation of these two amino acids is a likely indicator of leaf activated defense response. Furthermore, the amino acids glutamine37 and leucine38 have been reported to function as signaling molecules and to regulate important stress-responsive genes. Hence, amino acid profile changes may indicate a reprogramming of nitrogen metabolism to modulate carbon and nitrogen status, to manage plant growth or development or to stimulate defense upon exposure and/or stress.36
 | ||
| Fig. 3 Up-regulated and down-regulated amino acids in maize leaves in response to ENM exposure. Red, green, purple and blue represent the control, SiO2, TiO2 and Fe3O4, respectively. | ||
Additionally, the nitrogen-containing compound 4-aminobutyric acid (GABA) and its precursor (glutamic acid) significantly accumulated in maize leaves when grown in soil amended with ENMs (Fig. S2†). GABA is a non-protein amino acid that plays an essential role in signal transduction, pH regulation, nitrogen storage, plant development and stress defense.39 Here, we speculate that GABA may play a stress-signaling role in response to ENM exposure. Succinate semialdehyde (SSA), which is formed from GABA by transaminase, increased by up to 5–8 fold when compared to controls (Fig. S2†). SSA is a mitochondrially-generated intermediate in GABA metabolism and will accumulate intracellularly in response to a variety of biotic and abiotic stressors.40 Thus, the up-regulation of GABA and its up/down stream metabolites may indicate that the plant was able to sense root stress upon ENM exposure and the signal was transduced to the shoot tissues through signaling compounds.
In addition to GABA, a substantial increase of putrescine, another nitrogen-containing compound, was observed in maize leaves exposed to TiO2 (p < 0.05) and Fe3O4 (p < 0.01) (Fig. S2†). Putrescine is an important component of polyamines, which have also been reported to be involved in response to a variety of abiotic stresses.41 In addition to stress response, polyamines are involved in a number of physiological processes such as organogenesis, embryogenesis, floral initiation and development, leaf senescence and fruit development.42 Additionally, polyamine signaling is directly involved in different complex metabolic routes and intricate hormonal cross-talks.42 In addition, another polyamine known as spermine and its citrulline precursor were significantly up-regulated upon Fe3O4 ENM exposure. In summary, the up-regulation of GABA and a number of polyamines in maize may be a defense or coping strategy in response to ENM exposure.
Amino acid and carbohydrate metabolism are closely linked through glycolysis and the citric acid cycle (TCA cycle). In addition to amino acid profile alteration, a number of intermediates from glycolysis and the TCA cycle are precursors for amino acid biosynthesis, including pyruvic acid, oxaloacetate, and α-ketoglutarate. We observed that three TCA cycle intermediates, citric acid, alpha-ketoglutaric acid, and succinic acid were significantly up- or down-regulated in an ENM-dependent manner (Fig. S3†). This demonstrates that ENM exposure not only induced nitrogen metabolism perturbation, but also altered carbon metabolism.
Perturbed biological pathways in maize leaves
The significantly changed metabolites above are involved in a number of important metabolic pathways. The perturbed biological pathways were characterized using MetaboAnalyst 4.0. The results of this analysis reveal that there are 11 altered pathways in the leaves of maize plants exposed to Fe3O4 ENMs (Table S3,†Fig. 4A). It is noteworthy that a number of the disturbed pathways are related to nitrogen metabolism. In addition, carbohydrate-related pathways such as the TCA cycle, glycolysis and gluconeogenesis were also significantly altered by Fe3O4 exposure (Table S3†). The TCA cycle is a key pathway for the biosynthesis of plant hormones such as salicylic acid, ethylene, and auxin;43 TCA cycle intermediates are precursors for the synthesis of a variety of amino acids. The significant changes of TCA cycle metabolites suggest a significant metabolic reprogramming. Additionally, pyrimidine metabolism, which is involved in a number of important developmental processes such as germination, pollen tube growth, and flowering,44 was altered by Fe3O4 exposure. Inhibition of the pyrimidine pathway may be a strategy for the plant to re-allocate energy and resources for other stress-related processes.In contrast, TiO2 ENMs induced perturbations in 9 biological pathways, seven of which overlapped with Fe3O4 exposure (Table S3† and Fig. 4A). The TiO2-specific pathways were glycerophospholipid, glyoxylate and dicarboxylate metabolism, in which succinic acid and isocitric acid were significantly changed. In addition, SiO2 ENMs induced changes in 6 pathways (Table S3† and Fig. 4A). Importantly, there are three biological pathways commonly altered across all ENMs (Table S3† and Fig. 4A): arginine and proline metabolism, methane metabolism, and pantothenate and CoA biosynthesis. These metabolic pathway changes are central to nitrogen and carbohydrate metabolism.
Impact of ENMs on root metabolome
In maize root tissues, 360 metabolites were identified and semi-quantified by GC-MS. The PLS-DA loading plot (Fig. 2B) shows clear ENM-dependent separation from the untreated controls, particularly for Fe3O4 and TiO2, clearly suggesting metabolite profile alteration in below-ground tissues as well. The magnitude of metabolic changes followed the order Fe3O4 > TiO2 > SiO2, which shares the similar pattern to that in the leaves (Fig. 2A), and highlights the observation that Fe3O4 induced the most noticeable changes in exposed plant tissues. Meanwhile, a one-way ANOVA analysis revealed that only 7 metabolites (homovanillic acid, phosphate, D-glyceric acid, mannose, beta-hydroxypyruvate, ornithine and adenine, Fig. S4 and Table S2†) were significantly changed compared to the control, which is much less than 49 metabolites significantly changed in exposed leaf tissues. This observation that metabolic perturbation in the roots is less pronounced than that in the leaves is particularly interesting given that the roots were directly exposed to the ENM-amended soil. Across these metabolites, decreases in the simple polysaccharide mannose was found across all ENM treatments. An increase of mannose levels was reported in plants under cold stress.45 It has been reported that mannose governs the expression of the enzymatic antioxidant defense system.46 In addition, the up-regulation of phosphate, homovanillic acid, and D-glyceric acid and the down-regulation of ornithine and beta-hydroxypyruvate were Fe3O4-specific effects (Fig. S4 and Table S2†).Notably, Fe3O4 induced greater metabolic reprogramming than TiO2 and SiO2 NPs and a number of metabolites were only responsive to Fe3O4 exposure. For example, 1-hydroxyanthraquinone, 4-hydroxycinnamic acid, caffeic acid and ascorbate, which are reactive oxygen species scavengers, were significantly increased in Fe3O4-exposed root tissue (Fig. S5†). Moreover, phenylalanine, a precursor of antioxidant phenolic acids, was also up-regulated by Fe3O4. The up-regulation of antioxidant metabolites may indicate that Fe3O4 ENMs induced oxidative stress in maize roots. Our previous studies revealed that Cu(OH)2 NPs and Ag NPs also caused oxidative stress and upon exposure, plants activated low molecular weight antioxidant compounds to cope with this stress.47,48 In addition to the up-regulation of antioxidants, amino acids such as aspartic acid, lysine, serine and valine were also increased in response to Fe3O4 ENMs. Interestingly, serine and valine were also increased in maize leaves exposed to Fe3O4 ENMs. In addition, phenylalanine and tyrosine, which were increased in maize leaves exposed to Fe3O4 ENMs, were increased in the roots as well. As mentioned before, phenylalanine and tyrosine are precursors of defense related secondary metabolites. Their up-regulation in whole plant tissues indicates the activation of defensive systems.
Perturbed biological pathways in maize roots
A biological pathway analysis showed that there are only 4 pathways perturbed by Fe3O4 in the roots, which is significantly less than that in the leaves (11 pathways). As shown in Table S4† and Fig. 4B, the disturbed pathways include inositol phosphate metabolism, ascorbate and aldarate metabolism, glycerolipid metabolism and the TCA cycle. Interestingly, the TCA cycle is the only pathway which was significantly disrupted in both root and leaf tissues. Citric acid and isocitric acid, two important intermediates in the TCA cycle, were up-regulated in both tissues, indicating that energy-related metabolism was altered throughout the plant. A previous study has shown that both fatty acids and glycerolipid metabolism play an important role in plant defense.49 Ascorbate and aldarate metabolism is also well known as an antioxidant defense-related pathway. In addition, inositol phosphate functions as a secondary messenger for a variety of extracellular signals.50 Taken together, the activation of defense and antioxidant-related biological pathways clearly suggests that Fe3O4 induced a significant stress response.In contrast, TiO2 and SiO2 induced perturbation in five (metabolism of inositol phosphate, ascorbate/aldarate, methane, glyoxylate and dicarboxylate, and TCA cycle) and two (inositol phosphate metabolism and TCA cycle) biological pathways, respectively (Table S4† and Fig. 4B). It is noteworthy that inositol phosphate metabolism and TCA cycle are pathways that were perturbed in maize roots by all tested ENMs, indicating that carbohydrate metabolism is a sensitive target in root tissues, which is different from leaves where nitrogen and carbohydrate metabolism were the primary targets.
Soil metabolite profile
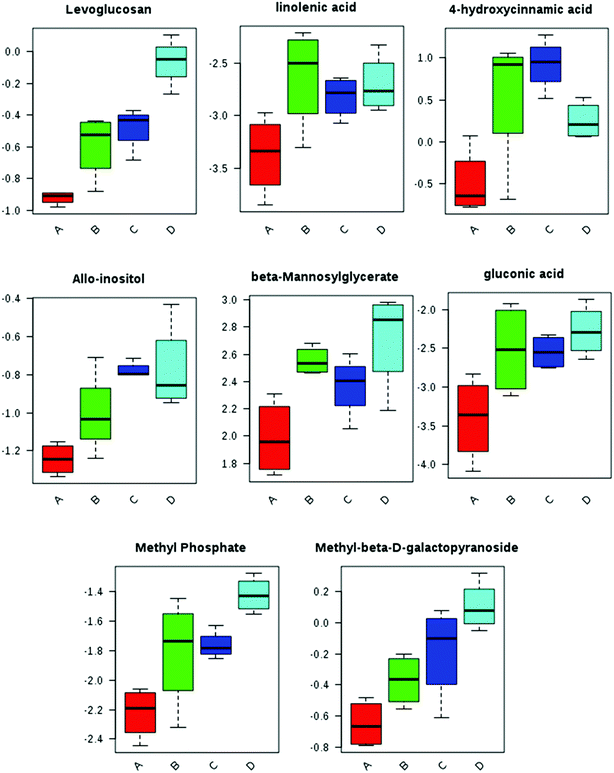
Rhizosphere chemistry is the result of root exuded chemicals, including their breakdown metabolites, as well as microbially released compounds and breakdown products.51 Root exudates are actively or passively secreted from root tissues and serve numerous functions to control abiotic and biotic processes, including changing the chemical and physical properties of the soil, inhibiting the growth of competing plants, combatting herbivores, and regulating the microbial community.52 GC-MS based metabolomics identified and semi-quantified 300 soil-derived metabolites, ranging from hydrophilic metabolites (e.g. sugar, organic acid, amino acids and nucleotides) to hydrophobic analytes (e.g. lipids and phenylpropanoids). The PLS-DA loading plot shows that the ENM groups (SiO2, TiO2, and Fe3O4) were clearly separated from the control group in a ENM-dependent manner along PC1 (Fig. 2C). This indicates that ENMs clearly altered the soil metabolite profile. Interestingly, Fe3O4 and TiO2 ENMs induced more pronounced metabolic response compared to SiO2 ENMs, which is similar to that in the plant leaf and root. Importantly, dissolved soil organic matter (DOM) is composed of small molecules of plant and microbial origin resulting from lysed cells and released metabolites.14 Thus, the altered soil metabolite profile may indicate altered DOM composition caused by ENM exposure, although the total content of DOM was unchanged (Fig. S6†).A one-way ANOVA analysis reveals that levoglucosan was significantly increased by 1.5–2 fold (p < 0.05) in planted soil amended with ENMs as compared to controls (Fig. 5 and Table S2†). Levoglucosan is a bioactive compound that is exuded into the rhizosphere, and has been associated with significantly increased root length in Arabidopsis. We speculate that levoglucosan may be acting as a signaling compound in soil in response to ENM exposure. In addition to levoglucosan, a number of metabolites, including linolenic acid, 4-hydroxycinnamic acid, allo-inositol, beta-mannosylglycerate, gluconic acid, methyl phosphate, and methyl-beta-D-galactopyranoside were all significantly (p < 0.05) increased by ENM amendment (Fig. 5 and Table S2†). These significantly modulated metabolites are classified as sugars, amino acids and amides, aliphatic and aromatic acids, and phenolics and fatty acids; all of which stimulate microbial growth or regulate plant growth.53 As mentioned above, exuded root metabolites play various roles in plant–plant, plant–microbe (including pathogens) and plant–pest interactions.52 For example, sugars and amino acids act as chemoattractants for other microbes.54 In addition, maize has been reported to up-regulate some metabolites, such as phenolics and flavonoids,55 to attract plant-beneficial rhizobacteria,56 including nitrogen fixing and growth promoting species.52 Zhalnina et al.57 observed that rhizosphere microbes showed affinity preference for the consumption of root secreted aromatic organic acids, such as nicotinic, salicylic and cinnamic acid. Conversely, some phenolic compounds (chlorogenic, caffeic and cinnamic acid) have been reported to enhance plant resistance to soilborne disease (Fusarium oxysporum f.sp. niveum).58 Dakora et al. demonstrated that phenolic compounds exuded by roots of N2-fixing legumes serve as primary signaling molecules to Rhizobia, bacteria which form root nodules and reduce nitrogen to ammonia.59 Therefore, actively selecting the released compounds may be part of a strategy plants employ to sense or cope with the ENMs-induced stress. Since these metabolites serve as carbon and energy sources for the microbial community, the altered soil metabolite profile or composition could subsequently impact soil microbial community composition. Further soil microbial analysis is needed to verify this hypothesis.
It must be noted that the observed soil metabolite changes are not completely the result of root exudated metabolites; the contribution from the soil microbial community cannot be neglected. Very likely, ENMs, especially those with antibacterial properties, can impact the soil microbial metabolic activity, and thereby trigger the up- or down-regulation of extracellular metabolite release. A number of ENMs have been reported to impact the soil microbial community structure and composition, including AgNPs,60,61 TiO2 and ZnO NPs.62 Thus, an altered soil metabolite profile may partially be attributed to passively released extracellular compounds by microbes. Unfortunately, the current experimental design does not enable elucidation of the relative contribution of the plant and microbes to the altered soil metabolites.
Conclusions
As the use of nanomaterials in the environment and nano-enabled agriculture continues to increase, a thorough understanding of their environmental impact will be critical to sustainable design and application. In this study, the response of both maize plants and the soil to engineered nanomaterials was investigated using a metabolomic strategy. The results provide a comprehensive perspective on molecular changes in the leaf, root and soil compartments as a function of ENM exposure. Importantly, although the exposure was in the soil, more pronounced metabolic changes were observed in plant leaves, highlighting the importance of in planta sensing and stress signaling originating in the root zone. The application of soil metabolomics enabled a single frame snapshot of soil chemical composition changes upon exposure to ENMs. This approach provides an integrated and simultaneous response of the soil and plant compartments. Our results demonstrate that soil metabolomics can be a powerful tool to identify the soil biotic responses to ENMs, and that this approach is transferrable to other contaminant groups. Additionally, this study observed the early response of maize seedlings to ENMs, and full life cycle studies are being planned to assess the impacts of ENMs on food yield and quality.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was funded by the National Key Research and Development Program of China under 2016YFD0800207 and National Natural Science Foundation of China under 21876081. JCW acknowledges USDA NIFA CONH 00147. Any opinions, findings, and conclusions or recommendations expressed in this material are those of authors and do not necessarily reflect the views of the National Science Foundation of China.References
- B. Fadeel, L. Farcal, B. Hardy, S. Vázquez-Campos, D. Hristozov, A. Marcomini, I. Lynch, E. Valsami-Jones, H. Alenius and K. Savolainen, Advanced tools for the safety assessment of nanomaterials, Nat. Nanotechnol., 2018, 13, 537–543 CrossRef CAS PubMed.
- Y. Ma, J. W. Metch, E. P. Vejerano, I. J. Miller, E. C. Leon, L. C. Marr, P. J. Vikesland and A. Pruden, Microbial community response of nitrifying sequencing batch reactors to silver, zero-valent iron, titanium dioxide and cerium dioxide nanomaterials, Water Res., 2015, 68, 87–97 CrossRef CAS PubMed.
- Y. Yang, J. Quensen, J. Mathieu, Q. Wang, J. Wang, M. Li, J. M. Tiedje and P. J. J. Alvarez, Pyrosequencing reveals higher impact of silver nanoparticles than Ag+ on the microbial community structure of activated sludge, Water Res., 2014, 48, 317–325 CrossRef CAS PubMed.
- J. W. Metch, N. D. Burrows, C. J. Murphy, A. Pruden and P. J. Vikesland, Metagenomic analysis of microbial communities yields insight into impacts of nanoparticle design, Nat. Nanotechnol., 2018, 13, 253–259 CrossRef CAS PubMed.
- Y. Ge, J. P. Schimel and P. A. Holden, Identification of Soil Bacteria Susceptible to TiO2 and ZnO Nanoparticles, Appl. Environ. Microbiol., 2012, 78, 6749–6758 CrossRef CAS PubMed.
- J. H. Priester, Y. Ge, R. E. Mielke, A. M. Horst, S. C. Moritz, K. Espinosa, J. Gelb, S. L. Walker, R. M. Nisbet and Y.-J. An, Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, E2451–E2456 CrossRef CAS PubMed.
- X.-F. Huang, J. M. Chaparro, K. F. Reardon, R. Zhang, Q. Shen and J. M. Vivanco, Rhizosphere interactions: root exudates, microbes, and microbial communities, Botany, 2014, 92, 267–275 CrossRef.
- D. V. Badri and J. M. Vivanco, Regulation and function of root exudates, Plant, Cell Environ., 2009, 32, 666–681 CrossRef CAS.
- D. Badri, G. Zolla, M. Bakker, D. Manter and J. M. Vivanco, Potential impact of soil microbiomes on the leaf metabolome and on herbivore feeding behavior, New Phytol., 2013, 198, 264–273 CrossRef CAS PubMed.
- N. W. Sokol and M. A. Bradford, Microbial formation of stable soil carbon is more efficient from belowground than aboveground input, Nat. Geosci., 2019, 12, 46–53 CrossRef CAS.
- M. Keiluweit, T. Wanzek, M. Kleber, P. Nico and S. Fendorf, Anaerobic microsites have an unaccounted role in soil carbon stabilization, Nat. Commun., 2017, 8, 1771 CrossRef PubMed.
- T. Ma, S. Zhu, Z. Wang, D. Chen, G. Dai, B. Feng, X. Su, H. Hu, K. Li, W. Han, C. Liang, Y. Bai and X. Feng, Divergent accumulation of microbial necromass and plant lignin components in grassland soils, Nat. Commun., 2018, 9, 3480 CrossRef PubMed.
- M. Kusano, A. Fukushima, H. Redestig and K. Saito, Metabolomic approaches toward understanding nitrogen metabolism in plants, J. Exp. Bot., 2011, 62, 1439–1453 CrossRef CAS PubMed.
- T. L. Swenson, S. Jenkins, B. P. Bowen and T. R. Northen, Untargeted soil metabolomics methods for analysis of extractable organic matter, Soil Biol. Biochem., 2015, 80, 189–198 CrossRef CAS.
- K. Zhalnina, K. B. Louie, Z. Hao, N. Mansoori, U. N. da Rocha, S. Shi, H. Cho, U. Karaoz, D. Loqué, B. P. Bowen, M. K. Firestone, T. R. Northen and E. L. Brodie, Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly, Nat. Microbiol., 2018, 3, 470–480 CrossRef CAS PubMed.
- O. A. H. Jones, S. Sdepanian, S. Lofts, C. Svendsen, D. J. Spurgeon, M. L. Maguire and J. L. Griffin, Metabolomic analysis of soil communities can be used for pollution assessment, Environ. Toxicol. Chem., 2014, 33, 61–64 CrossRef CAS PubMed.
- A. Weir, P. Westerhoff, L. Fabricius and N. von Goetz, Titanium Dioxide Nanoparticles in Food and Personal Care Products, Environ. Sci. Technol., 2012, 46, 2242–2250 CrossRef CAS PubMed.
- C. G. Athanassiou, N. G. Kavallieratos, G. Benelli, D. Losic, P. Usha Rani and N. Desneux, Nanoparticles for pest control: current status and future perspectives, J. Pestic. Sci., 2018, 91, 1–15 Search PubMed.
- R. Srivastava, Z. Li, G. Russo, J. Tang, R. Bi, U. Muppirala, S. Chudalayandi, A. Severin, M. He, S. I. Vaitkevicius, C. J. Lawrence-Dill, P. Liu, A. E. Stapleton, D. C. Bassham, F. Brandizzi and S. H. Howell, Response to Persistent ER Stress in Plants: A Multiphasic Process That Transitions Cells from Prosurvival Activities to Cell Death, Plant Cell, 2018, 30, 1220–1242 CrossRef CAS PubMed.
- D. M. Gates, Plant Photosynthetic Production. Manual of Methods. Z. Sestak, J. Catsky, P. G. Jarvis, Q. Rev. Biol., 1972, 47, 235–235 CrossRef.
- C. Larue, H. Castillo-Michel, S. Sobanska, N. Trcera, S. Sorieul, L. Cécillon, L. Ouerdane, S. Legros and G. Sarret, Fate of pristine TiO2 nanoparticles and aged paint-containing TiO2 nanoparticles in lettuce crop after foliar exposure, J. Hazard. Mater., 2014, 273, 17–26 CrossRef CAS PubMed.
- J. Xia, I. V. Sinelnikov, B. Han and D. S. Wishart, MetaboAnalyst 3.0—making metabolomics more meaningful, Nucleic Acids Res., 2015, 43, W251–W257 CrossRef CAS PubMed.
- Y. Jung, Y. G. Ahn, H. K. Kim, B. C. Moon, A. Y. Lee and G.-S. Hwang, Characterization of dandelion species using 1H NMR-and GC-MS-based metabolite profiling, Analyst, 2011, 136, 4222–4231 RSC.
- J. Xia and D. S. Wishart, MSEA: a web-based tool to identify biologically meaningful patterns in quantitative metabolomic data, Nucleic Acids Res., 2010, 38, W71–W77 CrossRef CAS PubMed.
- E. Kohan-Baghkheirati and J. Geisler-Lee, Gene Expression, Protein Function and Pathways of Arabidopsis thaliana Responding to Silver Nanoparticles in Comparison to Silver Ions, Cold, Salt, Drought, and Heat, Nanomaterials, 2015, 5, 436–467 CrossRef CAS PubMed.
- M. H. Ghafariyan, M. J. Malakouti, M. R. Dadpour, P. Stroeve and M. Mahmoudi, Effects of Magnetite Nanoparticles on Soybean Chlorophyll, Environ. Sci. Technol., 2013, 47, 10645–10652 CAS.
- M. Rui, C. Ma, Y. Hao, J. Guo, Y. Rui, X. Tang, Q. Zhao, X. Fan, Z. Zhang, T. Hou and S. Zhu, Iron Oxide Nanoparticles as a Potential Iron Fertilizer for Peanut (Arachis hypogaea), Front. Plant Sci., 2016, 7, 815 Search PubMed.
- Z. Zahra, M. Arshad, R. Rafique, A. Mahmood, A. Habib, I. A. Qazi and S. A. Khan, Metallic Nanoparticle (TiO2 and Fe3O4) Application Modifies Rhizosphere Phosphorus Availability and Uptake by Lactuca sativa, J. Agric. Food Chem., 2015, 63, 6876–6882 CrossRef CAS PubMed.
- R. Rafique, M. Arshad, M. Khokhar, I. A. Qazi, A. Hamza and N. Virk, Growth response of wheat to titania nanoparticles application, 2014 Search PubMed.
- V. L. Reddy Pullagurala, I. O. Adisa, S. Rawat, S. Kalagara, J. A. Hernandez-Viezcas, J. R. Peralta-Videa and J. L. Gardea-Torresdey, ZnO nanoparticles increase photosynthetic pigments and decrease lipid peroxidation in soil grown cilantro (Coriandrum sativum), Plant Physiol. Biochem., 2018, 132, 120–127 CrossRef CAS PubMed.
- P. S. Tourinho, C. A. M. van Gestel, S. Lofts, C. Svendsen, A. M. V. M. Soares and S. Loureiro, Metal-based nanoparticles in soil: Fate, behavior, and effects on soil invertebrates, Environ. Toxicol. Chem., 2012, 31, 1679–1692 CrossRef CAS PubMed.
- L. Vittori Antisari, S. Carbone, A. Gatti, G. Vianello and P. Nannipieri, Toxicity of metal oxide (CeO2, Fe3O4, SnO2) engineered nanoparticles on soil microbial biomass and their distribution in soil, Soil Biol. Biochem., 2013, 60, 87–94 CrossRef CAS.
- O. Shaul, Magnesium transport and function in plants: the tip of the iceberg, BioMetals, 2002, 15, 307–321 CrossRef.
- T. M. Bezemer and N. M. van Dam, Linking aboveground and belowground interactions via induced plant defenses, Trends Ecol. Evol., 2005, 20, 617–624 CrossRef PubMed.
- V. K. Rai, Role of Amino Acids in Plant Responses to Stresses, Biol. Plant., 2002, 45, 481–487 CrossRef CAS.
- R. Pratelli and G. Pilot, Regulation of amino acid metabolic enzymes and transporters in plants, J. Exp. Bot., 2014, 65, 5535–5556 CrossRef CAS PubMed.
- X. Liu and D. R. Bush, Expression and transcriptional regulation of amino acid transporters in plants, Amino Acids, 2006, 30, 113–120 CrossRef CAS PubMed.
- M. A. Hannah, C. Caldana, D. Steinhauser, I. Balbo, A. R. Fernie and L. Willmitzer, Combined Transcript and Metabolite Profiling of Arabidopsis Grown under Widely Variant Growth Conditions Facilitates the Identification of Novel Metabolite-Mediated Regulation of Gene Expression, Plant Physiol., 2010, 152, 2120–2129 CrossRef CAS PubMed.
- B. J. Shelp, A. W. Bown and M. D. McLean, Metabolism and functions of gamma-aminobutyric acid, Trends Plant Sci., 1999, 4, 446–452 CrossRef PubMed.
- W. L. Allan, K. E. Breitkreuz, J. C. Waller, J. P. Simpson, G. J. Hoover, A. Rochon, D. J. Wolyn, D. Rentsch, W. A. Snedden and B. J. Shelp, Detoxification of succinate semialdehyde in Arabidopsis glyoxylate reductase and NAD kinase mutants subjected to submergence stress, Botany, 2011, 90, 51–61 CrossRef.
- M. D. Groppa and M. P. Benavides, Polyamines and abiotic stress: recent advances, Amino Acids, 2007, 34, 35 CrossRef PubMed.
- R. Alcázar, T. Altabella, F. Marco, C. Bortolotti, M. Reymond, C. Koncz, P. Carrasco and A. F. Tiburcio, Polyamines: molecules with regulatory functions in plant abiotic stress tolerance, Planta, 2010, 231, 1237–1249 CrossRef PubMed.
- W.-L. Hung and Y. Wang, A Targeted Mass Spectrometry-Based Metabolomics Approach toward the Understanding of Host Responses to Huanglongbing Disease, J. Agric. Food Chem., 2018, 66(40), 10651–10661 CrossRef CAS PubMed.
- C. Kafer, L. Zhou, D. Santoso, A. Guirgis, B. Weers, S. Park and R. Thornburg, Regulation of pyrimidine metabolism in plants, 2004 Search PubMed.
- F. Chai, W. Liu, Y. Xiang, X. Meng, X. Sun, C. Cheng, G. Liu, L. Duan, H. Xin and S. Li, Comparative metabolic profiling of Vitis amurensis and Vitis vinifera during cold acclimation, Hortic. Res., 2019, 6, 8 CrossRef PubMed.
- A. Hameed, N. Iqbal and S. A. Malik, Mannose-Induced Modulations in Antioxidants, Protease Activity, Lipid Peroxidation, and Total Phenolics in Etiolated Wheat Leaves, J. Plant Growth Regul., 2009, 28, 58 CrossRef CAS.
- L. Zhao, Y. Huang, A. S. Adeleye and A. A. Keller, Metabolomics Reveals Cu(OH)2 Nanopesticide-Activated Anti-oxidative Pathways and Decreased Beneficial Antioxidants in Spinach Leaves, Environ. Sci. Technol., 2017, 51, 10184–10194 CrossRef CAS PubMed.
- H. Zhang, W. Du, J. R. Peralta-Videa, J. L. Gardea-Torresdey, J. C. White, A. Keller, H. Guo, R. Ji and L. Zhao, Metabolomics Reveals How Cucumber (Cucumis sativus) Reprograms Metabolites To Cope with Silver Ions and Silver Nanoparticle-Induced Oxidative Stress, Environ. Sci. Technol., 2018, 52(14), 8016–8026 CrossRef CAS PubMed.
- G.-H. Lim, R. Singhal, A. Kachroo and P. Kachroo, Fatty Acid– and Lipid-Mediated Signaling in Plant Defense, Annu. Rev. Phytopathol., 2017, 55, 505–536 CrossRef CAS PubMed.
- J. Mishra and U. S. Bhalla, Simulations of Inositol Phosphate Metabolism and Its Interaction with InsP3-Mediated Calcium Release, Biophys. J., 2002, 83, 1298–1316 CrossRef CAS PubMed.
- P. Pétriacq, A. Williams, A. Cotton, A. E. McFarlane, S. A. Rolfe and J. Ton, Metabolite profiling of non-sterile rhizosphere soil, Plant J., 2017, 92, 147–162 CrossRef PubMed.
- S. Rasmann and T. C. J. Turlings, Root signals that mediate mutualistic interactions in the rhizosphere, Curr. Opin. Plant Biol., 2016, 32, 62–68 CrossRef CAS PubMed.
- D. Faure, D. Vereecke and J. Leveau, Molecular communication in the rhizosphere, 2009 Search PubMed.
- E. Somers, J. Vanderleyden and M. Srinivasan, Rhizosphere Bacterial Signalling: A Love Parade Beneath Our Feet, Crit. Rev. Microbiol., 2004, 30, 205–240 CrossRef CAS PubMed.
- H. Massalha, E. Korenblum, D. Tholl and A. Aharoni, Small molecules below-ground: the role of specialized metabolites in the rhizosphere, Plant J., 2017, 90, 788–807 CrossRef CAS PubMed.
- A. L. Neal, S. Ahmad, R. Gordon-Weeks and J. Ton, Benzoxazinoids in Root Exudates of Maize Attract Pseudomonas putida to the Rhizosphere, PLoS One, 2012, 7, e35498 CrossRef CAS PubMed.
- K. Zhalnina, K. Louie, Z. Hao, N. Mansoori, U. Nunes da Rocha, S. Shi, H. Cho, U. Karaoz, D. Loqué, B. P. Bowen, M. K. Firestone, T. R. Northen and E. Brodie, Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly, 2018 Search PubMed.
- N. Ling, W. Zhang, D. Wang, J. Mao, Q. Huang, S. Guo and Q. Shen, Root Exudates from Grafted-Root Watermelon Showed a Certain Contribution in Inhibiting Fusarium oxysporum f. sp. niveum, PLoS One, 2013, 8, e63383 CrossRef CAS PubMed.
- F. D. Dakora and D. A. Phillips, in Food Security in Nutrient-Stressed Environments: Exploiting Plants' Genetic Capabilities, ed. J. J. Adu-Gyamfi, Springer Netherlands, Dordrecht, 2002, pp. 201–213, DOI:10.1007/978-94-017-1570-6_23.
- P. Das, C. J. Williams, R. R. Fulthorpe, M. E. Hoque, C. D. Metcalfe and M. A. Xenopoulos, Changes in Bacterial Community Structure after Exposure to Silver Nanoparticles in Natural Waters, Environ. Sci. Technol., 2012, 46, 9120–9128 CrossRef CAS PubMed.
- S. He, Y. Feng, J. Ni, Y. Sun, L. Xue, Y. Feng, Y. Yu, X. Lin and L. Yang, Different responses of soil microbial metabolic activity to silver and iron oxide nanoparticles, Chemosphere, 2016, 147, 195–202 CrossRef CAS PubMed.
- Y. Ge, J. P. Schimel and P. A. Holden, Evidence for Negative Effects of TiO2 and ZnO Nanoparticles on Soil Bacterial Communities, Environ. Sci. Technol., 2011, 45, 1659–1664 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Characteristics of ENMs (Table S1); significantly changed metabolites in maize tissues and soil exposure to different NPs (Table S2); perturbed biological pathways in leaf and root tissues (Tables S3 and S4); TEM images of SiO2 and Fe3O4 NPs (Fig. S1); the relative abundance of four nitrogen-containing compounds (Fig. S2); significantly changed carbohydrates in maize leaves in response to ENMs (Fig. S3); significantly changed metabolites in maize root by ENMs (Fig. S4); metabolites in maize roots that only respond to Fe3O4 ENMs (Fig. S5); total organic carbon content (Fig. S6). See DOI: 10.1039/c9en00137a |
| This journal is © The Royal Society of Chemistry 2019 |