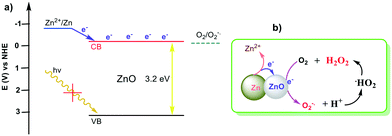
Redox active Zn/ZnO duo generating superoxide (˙O2−) and H2O2 under all conditions for environmental sanitation†
Guangshun
Yi
,
Xiukai
Li
,
Yuan
Yuan
and
Yugen
Zhang
 *
*
Institute of Bioengineering and Nanotechnology, 31 Biopolis Way, The Nanos #07-01, Singapore 138669, Singapore. E-mail: ygzhang@ibn.a-star.edu.sg
First published on 11th December 2018
Abstract
Photoactive semiconductors as bactericidal tools play an important role in various industries. These materials rely on the external stimulus of photoirradiation to generate reactive oxygen species (ROS), which limits their practical application to more general environments. Herein, a simple Zn/ZnO core/shell structure which continuously generates ROS without external energy input (in the dark) is developed. The core/shell structured Zn/ZnO particles showed excellent antibacterial activity when tested as surface coating, killing Gram-negative bacteria, Gram-positive bacteria and fungus, with more than 8-log cell reduction. Due to the spontaneously generated ROS, the Zn/ZnO core/shell particles can degrade dyes in the absence of light. Further study showed that the Zn/ZnO particles generate a high concentration of ˙O2− and H2O2 radicals which killed the bacteria and degraded the dyes. The generation of ROS without an external stimulus was proposed to be due to zinc corrosion and electron transfer processes. This new material provides a potential solution for general disinfection and environmental sanitation.
Environmental significanceAntimicrobial resistance (AMR) is one of the most critical challenges of our modern society. It was predicted that there could be more than 10 million deaths related to AMR in 2050. Common methods used to kill bacteria in the environment rely on organic disinfectants which may lead to secondary contamination and drug resistance. Photoactive semiconductors as bactericidal tools play an important role in various industries. These materials rely on the external stimulus of photoirradiation to generate reactive oxygen species (ROS), which limits their practical application in general environments. There is an urgent need to develop surface disinfection technology that is highly bactericidal but with no drug resistance issues and is benign to the environment. Herein, a simple Zn/ZnO core/shell structure is developed to continuously generate ROS in the absence of external energy input. The core/shell structured Zn/ZnO showed excellent antibacterial capability under all conditions, which killed Gram-negative bacteria, Gram-positive bacteria and fungus, with more than 8-log cell reduction. With their ROS generating properties, the Zn/ZnO core/shell particles can degrade dyes in the absence of light. Further study showed that Zn/ZnO generates a high concentration of ˙O2− and H2O2 radicals which killed the bacteria and degraded the dyes. The mechanism for the generation of ROS in the dark is proposed through zinc corrosion and electron transfer processes. This new material provides a potential solution to general disinfection and environmental sanitation. |
Semiconductor-based photocatalysts, which generate reactive oxygen species (ROS) under photoexcitation, have a broad range of applications as antimicrobial agents in health care, consumer care, and food and water industries.1–5 ROS also play important roles in various physiological processes.6 Current efforts on semiconductor photocatalysts are devoted to increasing their photocatalytic efficiency by doping semiconductors with various metals and non-metals to speed up the electron–hole pair separation and narrow the energy band gap for harvesting visible light.7–9 Semiconductor photocatalysts are also modified with conductive polymers for temporary electron storage.10–13 A crucial limitation of the current systems for ROS generation is the requirement of an external stimulus, such as photoirradiation, thereby limiting the ROS generation strength and thwarting their practical application. With this concern, a simple system that can generate ROS without external energy input is highly desirable.
ZnO is one of the most important semiconducting metal oxides with a wide band gap energy of 3.2–3.4 eV and large exciton binding energy (60 meV).1 ZnO nanomaterials are considered as excellent candidates for photocatalytic antimicrobial agents since they generate ROS which eventually oxidize organic materials and thus impart biocidal properties to ZnO.12,14–19 However, these properties rely on external photoirradiation, and the photoenergy conversion efficiency of ZnO is low due to the relatively low charge separation efficiency and fast recombination of charge carriers. Recently, it has been reported that metals can donate electrons to metal oxide semiconductors through a self-corrosion process in Mg–TiO2 systems.20 Similarly, this self-corrosion process could also happen on Zn/ZnO composites (Scheme 1). The electrons generated from zinc corrosion can be transferred into the conduction band (CB) of ZnO in an energetically favorable way. The electrons in the CB are able to reduce oxygen and generate ROS.1 In other words, the electrons are donated from Zn to ZnO (CB) and reduce oxygen molecules to generate radicals in an energetically favorable way.17,21 The whole system does not rely on an external stimulus and the ROS generation process could be manipulated and have long-term stability. New materials designed based on this concept could play pivotal roles as non-toxic and safe antimicrobial technology to replace organic disinfectants, antiseptics and antibiotics in a broad range of applications, especially in the control of infectious disease and antimicrobial resistance (AMR) transmission.
Due to the increasing concerns on chemical toxicity, drug resistance development, environmental burdens, and food chain contamination caused by the overuse of organic disinfectants/antibiotics, inorganic-based disinfectants have attracted more and more attention.22 Compared with organic-based disinfectants, ROS-releasing inorganic materials are more stable and less toxic, although they are less efficient and rely on external stimuli. Herein, we report Zn/ZnO core/shell structured particles which can continuously release a high concentration of ROS by self-corrosion without any external stimulus. The new materials have excellent antibacterial properties as well as dye degradation capability.
To prove this concept, the conjugation of Zn and ZnO particles was firstly tested for their antibacterial properties. As shown in Fig. S1,† glass surfaces with dimensions of 2.5 cm × 2.5 cm were coated with Zn particles (0.02 g, 1–10 μm), or ZnO particles (0.02 g, 0.2–0.5 μm), and a smooth and densely coated surface was formed. The antibacterial efficacy of the surfaces was evaluated based on the Japanese Industrial Standard method (JIS Z 2801/ISO 22196 for antimicrobial product tests).23 This method is well-recognized in industry for the assessment of antibacterial surfaces. As shown in Fig. S1,† the surfaces coated with Zn or ZnO did not show antibacterial activities against E. coli. In contrast, the glass surface coated with a mixture of Zn (0.01 g) and ZnO (0.01 g) showed strong antibacterial activity. Importantly, all these tests were carried out in the dark without an external stimulus.
This experiment clearly showed that the combination/conjugation of Zn and ZnO has significantly enhanced the antibacterial properties. Based on this, core/shell structured Zn/ZnO which has an ideal Zn and ZnO interface was designed, prepared and its antibacterial properties were evaluated.
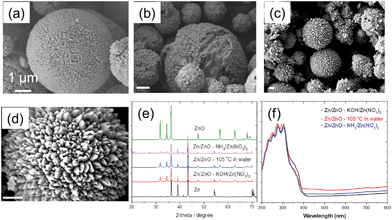
Fig. 1a shows the formation of the Zn/ZnO core/shell structure prepared from the reaction of micron-sized Zn particles with [Zn(OH)4]2− in a base solution (method A) resulting in the growth of ZnO nanopillars on the surface of the Zn core.24,25 The high-resolution TEM (HRTEM) image of the pillars in Fig. S2† reveals the crystalline structure of ZnO. The preferential growth direction of the pillar-like ZnO crystal is along the [001] direction. The displayed 0.26 nm lattice spacing corresponds to the lattice spacing of the wurtzite ZnO (002) plane.25 The size of the Zn core is in the range of 1–10 μm which is consistent with the size of the original Zn particles. The size of ZnO pillars on the shell is in the range of 0.2–0.5 μm, similar to the size range of the ZnO particles shown in Fig. S1.† Similarly, the Zn/ZnO core/shell structure was also obtained by the reaction of Zn particles in [Zn(NH4)4]2− aqueous solution at 100 °C (method B),26 as shown in Fig. 2b. The Zn particles were partially covered by the ZnO nanopillars. XRD showed impurity peaks between 20° and 30°, which belong to Zn(OH)2. The intensity of the ZnO peak is weaker compared to that of method A Zn/ZnO particles, which indicated that less ZnO was formed on the Zn core.
Different from the methods mentioned above, by the direct reaction of Zn particles with water in a sealed reactor at 105 °C (method C), a durian-like core/shell structure of Zn/ZnO was obtained, as shown in Fig. 1(c and d). The surface of the Zn core was fully covered with wedge-shaped ZnO.
The XRD patterns of the three prepared samples in Fig. 1e show the appearance of both Zn and ZnO diffraction peaks, which confirmed the co-existence of Zn and ZnO in the particles. No other impurity except Zn(OH)2 was found in Zn/ZnO–NH3/Zn(NO3)2 particles. The intensity ratios of I(100)/I(002) of ZnO are all less than 1.27
The optical absorption of the three samples in the UV-visible (UV-vis) range was recorded and is shown in Fig. 1f. All the samples show obvious, unimodal, and intense absorption within 200–400 nm, where the method A Zn/ZnO particle has broader absorption with a clear red-shift, indicating a narrower energy gap (CB/VB). However, this difference is proved to be not critical to its antibacterial properties.
The antibacterial properties of the three types of Zn/ZnO core/shell structures synthesized under different conditions were evaluated against E. coli. All of the samples exhibited antibacterial activity. The samples synthesized using methods A and C demonstrated excellent bactericidal properties which killed all E. coli after 24 h of incubation, with log reduction >8 (Fig. S3†). Zn/ZnO synthesized using method B (Fig. 1b) showed 3.4-log cell reduction, which is also bactericidal according to the JIS standard (>2), although it is less effective than the other samples (Fig. S3†). The lower antibacterial properties could be due to the impurity and low crystallinity of the ZnO layer, as indicated in the XRD pattern. In comparison, the single component of ZnO nanopillars separated from the core/shell structure has no antimicrobial activity under the same conditions (Fig. S4†).28,29
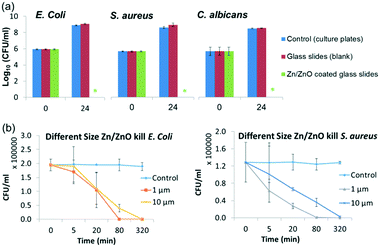
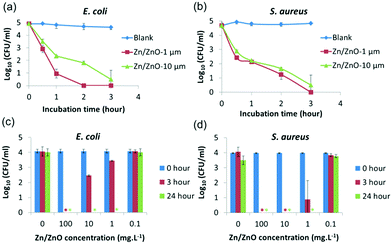
The antibacterial properties of the selected core/shell Zn/ZnO particles synthesized under method A were systematically evaluated with the same method against E. coli (ATCC 8739), S. aureus (ATCC 6538P) and C. albicans (ATCC 10231). As shown in Fig. 2, the Zn/ZnO particle-coated surfaces were antimicrobial (Fig. 2a), with 8-log reduction for E. coli and S. aureus, respectively, after 24 hours of incubation. C. albicans, as an example of a fungus, was also tested and all the fungal cells were killed after 24 hours of incubation. Remarkably, Zn/ZnO particles stored in air for 2 years exhibited the same antimicrobial activity as the newly prepared ones.
The structure of the Zn/ZnO core/shell particles could be tuned by using zinc precursors with different sizes or changing the reaction conditions (such as the reaction time and the concentration of zinc salt to adjust the thickness of the ZnO layer). The morphology of the ZnO shell will also be changed from nano-pillars to urchin-like microspheres when the concentrations of the reagents decrease (see Fig. S5†).28 In general, Zn/ZnO particles with smaller size have better antibacterial performance and faster killing speed (Fig. 2b). This is due to the larger surface area (17.4 m2 g−1vs. 5.8 m2 g−1) of the smaller particles which could produce more active species.
The antibacterial activity of core/shell Zn/ZnO was further studied by the disk diffusion assay against E. coli and S. aureus.30 The size of inhibition zones depends on the active species released from the tested sample into the aqueous agar matrix. The Zn/ZnO core/shell particles exhibited the biggest inhibition zone compared to the Zn powder, ZnO particles and the mixture of the two (Table S1†). This result indicates that Zn/ZnO releases more active species into the surrounding area, which are most likely to be Zn2+ (ref. 31) and/or reactive oxygen species (ROS).15,17,30,32 Therefore, the amount of released Zn2+ and ROS in the solution was measured to identify the antibacterial mechanism.
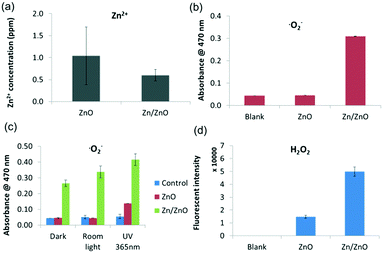
Fig. 3a shows the concentration of Zn2+ leached from ZnO particles and Zn/ZnO particles in water after 24 hours of incubation. Zn/ZnO releases less Zn2+ than ZnO in DI water and in other sources of water (reservoir water and sea water). The concentrations of Zn2+ released from both Zn/ZnO and ZnO are far less than the MIC of Zn2+ (Table S2†). Apparently, the release of Zn2+ is not the dominant factor for killing the bacteria.
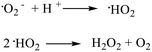
For the release of ROS, both OH˙ and ˙O2− radicals were monitored. There was no significant difference in the OH˙ radical concentration in ZnO, Zn/ZnO and the blank control (Fig. S6†).33Fig. 3b clearly shows that Zn/ZnO releases a much higher level of ˙O2− in the dark, while the amount of ˙O2− released from ZnO is similar to that from the blank control.5,34 Apparently, Zn/ZnO can generate a high level of superoxide radicals in the dark while ZnO cannot. The generation of ˙O2− was also confirmed using the nitroblue tetrazolium (NBT) method35 (Fig. S7†). To further understand the mechanism of ROS generation, the ROS levels in the dark, under room light and under UV were also tested (Fig. 3c and S8†). As shown in Fig. 3c, the concentrations of ˙O2− radicals released from Zn/ZnO under room light and UV increase by 20% and 50% compared to that in the dark. Importantly, the amount of ˙O2− radicals released from Zn/ZnO in the dark is two times that released by ZnO under UV irradiation. This result indicates that the generation of ˙O2− from Zn/ZnO is not dominated by the traditional light-driven electron–hole separation model.
Interestingly, both ZnO and Zn/ZnO can generate H2O2, while Zn/ZnO generates a nearly 3 times higher level of H2O2 than ZnO as shown in Fig. 3d.36 The existence of H2O2 and the higher level of H2O2 in the Zn/ZnO system were also confirmed by the phenol red assay (Fig. S9†).37 The generation of H2O2 may come from two sources. On the one hand, ZnO itself may generate H2O2 through surface oxygen vacancies,32,36,38 as shown in Fig. 3c. On the other hand, the high level of H2O2 from the Zn/ZnO system may be converted from the ˙O2− radicals, through the following reaction (Scheme 2).32,39
Therefore, it could be concluded that the higher antibacterial activity of the Zn/ZnO core/shell particles is related to their capability to generate ROS (˙O2− and H2O2), rather than the release of Zn2+. It should be noted that all the experiments including the antibacterial tests were conducted in the dark, which means that the ROS are generated from the Zn/ZnO core/shell particles without photoirradiation. This is in sharp contrast to the current photocatalytic semiconductors, such as TiO2 or ZnO,10–12 which generate ROS by photoirradiation, and opens a new window for advanced antimicrobial technology.
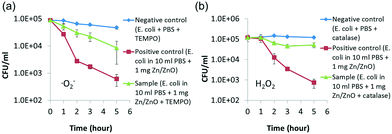
To further verify the ROS killing mechanism, ROS scavenging experiments were carried out. TEMPO was used to capture and quench the ˙O2− radicals, while catalase was used to capture and quench H2O2.35 As shown in Fig. 4, the Zn/ZnO core/shell particles kill the bacteria much faster in the solution without the scavenger than in the solution with the scavenger. This showed that the ROS is the dominant factor for killing the bacteria in our experiments. It was also found from Fig. 4 that both ˙O2− and H2O2 contribute significantly to the antibacterial properties of Zn/ZnO.
It is clear that the microbicidal properties of the Zn/ZnO core/shell particles rely on the high levels of ˙O2− and H2O2 which are generated from the redox active Zn/ZnO system. The superior antimicrobial properties of the Zn/ZnO core/shell particles certainly have many potential applications, both in vitro and in vivo. Concerning the biosafety, the hemolytic activity of our Zn/ZnO particles was examined by incubating rat red blood cells (rRBCs) with various concentrations of the antimicrobial agents (Fig. S10†). The Zn/ZnO particles induced negligible hemolysis even at concentrations up to 2000 μg ml−1. The Zn/ZnO core/shell particles are also negative for mutagenicity in the Ames test, which means that the Zn/ZnO particles have no genotoxicity or mutagenic potential (Table S3†).
Safe and clean drinking water is a basic requirement for human beings. However, due to the fast increase in population and industrial pollution, clean drinking water becomes a global challenge. Organics and pathogens are the two major pollutants in water which threaten public health. Chlorine is widely used for the removal of these contaminants in water due to its potency and low cost.40 However, it is always accompanied by risks, such as carcinogenic by-products and secondary pollutants. Although alternative disinfection processes such as advanced filtration and germicidal ultraviolet (UV) radiation have been developed, they are usually energy intensive. Semiconductor photocatalysts including TiO2, ZnO, and modified MoS2 nanofilms35 have been well documented for water disinfection and organics degradation. However, all these materials rely on external stimuli, such as photoirradiation. Herein, water disinfection using redox active Zn/ZnO was tested under dark conditions.
To test how fast the bacteria can be killed, Zn/ZnO was added into water which contains 105 CFU ml−1 bacteria. The solution was kept in the dark with agitation. The bacteria concentration in the solution was monitored by the plate counting technique hourly. As shown in Fig. 5, nearly all of E. coli and S. aureus were killed within 3 hours. It was also found that Zn/ZnO with smaller size (1 μm) kills the bacteria faster than the one with bigger size (1–10 μm), which is reasonable as the smaller particles release more ROS than the bigger particles. To test the minimum concentration of Zn/ZnO for effective water disinfection, Zn/ZnO (1 μm) with different concentrations was added into water containing 105 CFU ml−1 bacteria. The surviving bacteria in the solution after 3 h and 24 h were analyzed. It showed that 1 mg L−1 Zn/ZnO can kill 95% of E. coli at 3 h and no surviving E. coli was observed at 24 h. Meanwhile 1 mg L−1 Zn/ZnO can kill more than 99.9% of S. aureus at 3 h and no surviving cells were detected at 24 h. We conclude that the Zn/ZnO core/shell particles are able to kill bacteria effectively in water at 1 mg L−1 without the presence of light.40,41
Besides bacterial pollutants in water, dyes and other organics may cause water pollution as well and they can be decomposed by ROS from photocatalysts, such as TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 and ZnO8,12,42 in the presence of UV or sunlight.3,43 Herein, methylene blue, as an example of a pollutant in aqueous solution, was co-cultured with ZnO and Zn/ZnO, respectively. As shown in Fig. S11,† the dye was decomposed by the Zn/ZnO core/shell particles at room temperature in the dark or under room light. In comparison, no degradation was observed for the dye cultured with ZnO. Furthermore, Zn/ZnO can be reused. The recycled Zn/ZnO exhibits the same dye degradation activity and radical release properties (Fig. S11c and d†). It means that Zn/ZnO generates ROS continuously in the dark and has long-term stability.
41 and ZnO8,12,42 in the presence of UV or sunlight.3,43 Herein, methylene blue, as an example of a pollutant in aqueous solution, was co-cultured with ZnO and Zn/ZnO, respectively. As shown in Fig. S11,† the dye was decomposed by the Zn/ZnO core/shell particles at room temperature in the dark or under room light. In comparison, no degradation was observed for the dye cultured with ZnO. Furthermore, Zn/ZnO can be reused. The recycled Zn/ZnO exhibits the same dye degradation activity and radical release properties (Fig. S11c and d†). It means that Zn/ZnO generates ROS continuously in the dark and has long-term stability.
Many dyes are known to be adsorbed strongly on metal oxide surfaces. To verify that the decrease of UV-vis absorbance in the methylene blue degradation experiment is due to the degradation process by ROS, methylene blue aqueous solution was cultured with Zn/ZnO and the mixture of Zn/ZnO and TEMPO, which was added as the ˙O2− scavenger.35 As shown in Fig. S12,† in the reactor with methylene blue and Zn/ZnO, the absorbance was continually decreasing. However, when TEMPO was added, the absorbance of methylene blue did not decrease and remained constant. This experiment proved that the change of UV absorbance is due to the degradation of methylene blue with ˙O2− from Zn/ZnO. Besides methylene blue, other dyes such as Rhodamine B and phenol red were also tested. They however cannot be degraded under the same conditions. This may be due to the ˙O2− radical having lower oxidation power as compared with the hydroxyl radical.
To create non-toxic disinfecting surfaces in healthcare or public settings with long-term activity and stability, Zn/ZnO core/shell particles were used as antibacterial additives in polyacrylic acid (PAA) emulsion paint which is commonly used on various surfaces. Commercial Nippon paints were mixed with different concentrations of Zn/ZnO core/shell particles (0%, 1%, 3% and 5%, wt%), coated onto the testing surface (polycarbonates, 5 cm × 5 cm) and evaluated using the JIS Z 2801 method. The blank polycarbonate samples with and without Nippon paint coating were used as controls. As shown in Fig. S13,† for E. coli, the surface became bactericidal (2-log reduction) when the paint was mixed with 3% Zn/ZnO and all E. coli were killed (>9-log reduction) when 5% Zn/ZnO doping was used. For S. aureus, the painted surface became bactericidal (2-log reduction) when 1% Zn/ZnO was added in the paint, and all S. aureus were killed when 3% Zn/ZnO doping was used in the paint. The paint without Zn/ZnO is not antibacterial for both bacteria. This experiment showed that our core/shell structured Zn/ZnO can be used as an antibacterial agent in surface painting.
In summary, redox active Zn/ZnO core/shell structured particles have been proven to have superb antimicrobial properties without any external stimuli. The Zn/ZnO particles generate high levels of superoxide and H2O2 radicals through a self-corrosion process which contributes to their antimicrobial activity. The Zn/ZnO particles demonstrate much stronger capacity for generating ROS compared to various photo-catalytic semiconductors, yet they do not rely on photoirradiation. Applications of the Zn/ZnO particles in water disinfection, dye degradation and surface disinfection have been demonstrated. Considering their safety and long-term stability, the Zn/ZnO particles could be a promising non-toxic antimicrobial material to replace organic disinfectants, antiseptics and antibiotics in a broad range of environmental sanitation applications.
This work was supported by the Institute of Bioengineering and Nanotechnology, Biomedical Research Council, Agency for Science, Technology and Research and the National Research Foundation, Prime Minister's Office, Singapore under its NRF Competitive Research Program (NRF-CRP19-2017-02).
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Janotti and C. G. Van de Walle, Fundamentals of zinc oxide as a semiconductor, Rep. Prog. Phys., 2009, 72, 1–29 CrossRef
.
- M. N. Chong, B. Jin, C. W. K. Chow and C. Saint, Recent developments in photocatalytic water treatment technology: A review, Water Res., 2010, 44, 2997–3027 CrossRef CAS PubMed
.
- S. Dong, J. Feng, M. Fan, Y. Pi, L. Hu, X. Han, M. Liu, J. Sun and J. Sun, Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: a review, RSC Adv., 2015, 5, 14610–14630 RSC
.
- Q. Li, R. Xie, Y. W. Ll, E. A. Mintz and J. K. Shang, Enhanced visible-light-induced photocatalytic disinfection of E-coli by carbon-sensitized nitrogen-doped titanium oxide, Environ. Sci. Technol., 2007, 41, 5050–5056 CrossRef CAS PubMed
.
- Y. Li, W. Zhang, J. Niu and Y. Chen, Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles, ACS Nano, 2012, 6, 5164–5173 CrossRef CAS PubMed
.
- A. J. Able, D. I. Guest and M. W. Sutherland, Use of a New Tetrazolium-Based Assay to Study the Production of Superoxide Radicals by Tobacco Cell Cultures Challenged with Avirulent Zoospores of Phytophthora parasitica var nicotianae, Plant Physiol., 1998, 117, 491–499 CrossRef CAS PubMed
.
- Y. Ben-Shahar, J. P. Philbin, F. Scotognella, L. Ganzer, G. Cerullo, E. Rabani and U. Banin, Charge Carrier Dynamics in Photocatalytic Hybrid Semiconductor–Metal Nanorods: Crossover from Auger Recombination to Charge Transfer, Nano Lett., 2018, 18, 5211–5216 CrossRef CAS PubMed
.
- N. S. Han, D. Kim, J. W. Lee, J. Kim, H. S. Shim, Y. Lee, D. Lee and J. K. Song, Unexpected Size Effect Observed in ZnO-Au Composite Photocatalysts, ACS Appl. Mater. Interfaces, 2016, 8, 1067–1072 CrossRef CAS PubMed
.
- M. Zhao, H. Xu, S. Ouyang, D. Li, X. Meng and J. Ye, Effect of band structure on the hot-electron transfer over Au photosensitized brookite TiO2, Phys. Chem. Chem. Phys., 2016, 18, 3409–3412 RSC
.
- C. Dette, M. A. Perez-Osorio, C. S. Kley, P. Punke, C. E. Patrick, P. Jacobson, F. Giustino, S. J. Jung and K. Kern, TiO2 Anatase with a Bandgap in the Visible Region, Nano Lett., 2014, 14, 6533–6538 CrossRef CAS PubMed
.
- J. Xu, X. Zhou, Z. Gao, Y.-Y. Song and P. Schmuki, Visible-Light-Triggered Drug Release from TiO2 Nanotube Arrays: A Controllable Antibacterial Platform, Angew. Chem., Int. Ed., 2016, 55, 593–597 CrossRef CAS PubMed
.
- W. He, H.-K. Kim, W. G. Wamer, D. Melka, J. H. Callahan and J.-J. Yin, Photogenerated Charge Carriers and Reactive Oxygen Species in ZnO/Au Hybrid Nanostructures with Enhanced Photocatalytic and Antibacterial Activity, J. Am. Chem. Soc., 2014, 136, 750–757 CrossRef CAS PubMed
.
- F. Kayaci, S. Vempati, C. Ozgit-Akgun, N. Biyikli and T. Uyar, Enhanced photocatalytic activity of homoassembled ZnO nanostructures on electrospun polymeric nanofibers: A combination of atomic layer deposition and hydrothermal growth, Appl. Catal., B, 2014, 156–157, 173–183 CrossRef CAS
.
- P. J. P. Espitia, N. d. F. F. Soares, J. S. d. R. Coimbra, N. J. de Andrade, R. S. Cruz and E. A. A. Medeiros, Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications, Food Bioprocess Technol., 2012, 5, 1447–1464 CrossRef CAS
.
- G. Applerot, A. Lipovsky, R. Dror, N. Perkas, Y. Nitzan, R. Lubart and A. Gedanken, Enhanced Antibacterial Activity of Nanocrystalline ZnO Due to Increased ROS-Mediated Cell Injury, Adv. Funct. Mater., 2009, 19, 842–852 CrossRef CAS
.
- U. Kadiyala, E. S. Turali-Emre, J. H. Bahng, N. A. Kotov and J. S. VanEpps, Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA), Nanoscale, 2018, 10, 4927–4939 RSC
.
- A. Sirelkhatim, S. Mahmud, A. Seeni, N. H. M. Kaus, L. C. Ann, S. K. M. Bakhori, H. Hasan and D. Mohamad, Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism, Nano-Micro Lett., 2015, 7, 219–242 CrossRef CAS PubMed
.
- Y. Xie, Y. He, P. L. Irwin, T. Jin and X. Shi, Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni, Appl. Environ. Microbiol., 2011, 77, 2325–2331 CrossRef CAS PubMed
.
- S. Podder, S. Halder, A. Roychowdhury, D. Das and C. K. Ghosh, Superb hydroxyl radical-mediated biocidal effect induced antibacterial activity of tuned ZnO/chitosan type II heterostructure under dark, J. Nanopart. Res., 2016, 18, 294–306 CrossRef
.
- J. Park, P. Du, J.-K. Jeon, G. H. Jang, M. P. Hwang, H.-S. Han, K. Park, K. H. Lee, J.-W. Lee, H. Jeon, Y.-C. Kim, J. W. Park, H.-K. Seok and M.-R. Ok, Magnesium Corrosion Triggered Spontaneous Generation of H2O2 on Oxidized Titanium for Promoting Angiogenesis, Angew. Chem., Int. Ed., 2015, 54, 14753–14757 CrossRef CAS PubMed
.
- P. Nagarajan and V. Rajagopalan, Enhanced bioactivity of ZnO nanoparticles—an antimicrobial study, Sci. Technol. Adv. Mat., 2008, 9, 035004 CrossRef PubMed
.
- World Health Organization, Antimicrobial resistance: global report on surveillance, 2014, p. 257 Search PubMed.
- Japanese Standards Association, Antimicrobial products test for antimicrobial activity and efficacy, JIS Z 2801, 2000, Japan, 2001-08 Search PubMed.
- G. Yi, Y. Yuan, X. Li and Y. Zhang, ZnO Nanopillar Coated Surfaces with Substrate-Dependent Superbactericidal Property, Small, 2018, 14, e1703159 CrossRef PubMed
.
- X. F. Wu, H. Bai, C. Li, G. W. Lu and G. Q. Shi, Controlled one-step fabrication of highly oriented ZnO nanoneedle/nanorods arrays at near room temperature, Chem. Commun., 2006, 1655–1657 RSC
.
- O. Lupan, L. Chow, G. Chai and H. Heinrich, Fabrication and characterization of Zn–ZnO core–shell microspheres from nanorods, Chem. Phys. Lett., 2008, 465, 249–253 CrossRef CAS
.
- X. Liu, L. Ye, S. Liu, Y. Li and X. Ji, Photocatalytic Reduction of CO2 by ZnO Micro/nanomaterials with Different Morphologies and Ratios of {0001} Facets, Sci. Rep., 2016, 6, 38474 CrossRef CAS PubMed
.
- Q. Cai, Y. Gao, T. Gao, S. Lan, O. Simalou, X. Zhou, Y. Zhang, C. Harnoode, G. Gao and A. Dong, Insight into Biological Effects of Zinc Oxide Nanoflowers on Bacteria: Why Morphology Matters, ACS Appl. Mater. Interfaces, 2016, 8, 10109–10120 CrossRef CAS PubMed
.
- X. Wang, F. Yang, W. Yang and X. Yang, A study on the antibacterial activity of one-dimensional ZnO nanowire arrays: effects of the orientation and plane surface, Chem. Commun., 2007, 4419–4421 RSC
.
- Y.-W. Wang, A. Cao, Y. Jiang, X. Zhang, J.-H. Liu, Y. Liu and H. Wang, Superior Antibacterial Activity of Zinc Oxide/Graphene Oxide Composites Originating from High Zinc Concentration Localized around Bacteria, ACS Appl. Mater. Interfaces, 2014, 6, 2791–2798 CrossRef CAS PubMed
.
- J. Pasquet, Y. Chevalier, J. Pelletier, E. Couval, D. Bouvier and M.-A. Bolzinger, The contribution of zinc ions to the antimicrobial activity of zinc oxide, Colloids Surf., A, 2014, 457, 263–274 CrossRef CAS
.
- V. Lakshmi Prasanna and R. Vijayaraghavan, Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark, Langmuir, 2015, 31, 9155–9162 CrossRef CAS PubMed
.
- S. Xiong, S. George, Z. Ji, S. Lin, H. Yu, R. Damoiseaux, B. France, K. W. Ng and S. C. J. Loo, Size of TiO2 nanoparticles influences their phototoxicity: an in vitro investigation, Arch. Toxicol., 2013, 87, 99–109 CrossRef CAS PubMed
.
- Y. A. Nor, H. W. Zhang, S. Purwajanti, H. Song, A. K. Meka, Y. Wang, N. Mitter, D. Mahony and C. Z. Yu, Hollow mesoporous carbon nanocarriers for vancomycin delivery: understanding the structure-release relationship for prolonged antibacterial performance, J. Mater. Chem. B, 2016, 4, 7014–7021 RSC
.
- C. Liu, D. Kong, P.-C. Hsu, H. Yuan, H.-W. Lee, Y. Liu, H. Wang, S. Wang, K. Yan, D. Lin, P. A. Maraccini, K. M. Parker, A. B. Boehm and Y. Cui, Rapid water disinfection using vertically aligned MoS2 nanofilms and visible light, Nat. Nanotechnol., 2016, 11, 1098–1104 CrossRef CAS PubMed
.
- K. H. Tam, A. B. Djurišić, C. M. N. Chan, Y. Y. Xi, C. W. Tse, Y. H. Leung, W. K. Chan, F. C. C. Leung and D. W. T. Au, Antibacterial activity of ZnO nanorods prepared by a hydrothermal method, Thin Solid Films, 2008, 516, 6167–6174 CrossRef CAS
.
- B. Sels, D. D. Vos, M. Buntinx, F. Pierard, A. Kirsch-De Mesmaeker and P. Jacobs, Layered double hydroxides exchanged with tungstate as biomimetic catalysts for mild oxidative bromination, Nature, 1999, 400, 855–857 CrossRef CAS
.
- F. Kayaci, S. Vempati, I. Donmez, N. Biyikli and T. Uyar, Role of zinc interstitials and oxygen vacancies of ZnO in photocatalysis: a bottom-up approach to control defect density, Nanoscale, 2014, 6, 10224–10234 RSC
.
- F. Kayaci, S. Vempati, C. Ozgit-Akgun, I. Donmez, N. Biyikli and T. Uyar, Selective isolation of the electron or hole in photocatalysis: ZnO-TiO2 and TiO2-ZnO core-shell structured heterojunction nanofibers via electrospinning and atomic layer deposition, Nanoscale, 2014, 6, 5735–5745 RSC
.
- H. Bai, Z. Liu and D. D. Sun, Hierarchical ZnO/Cu “corn-like” materials with high photodegradation and antibacterial capability under visible light, Phys. Chem. Chem. Phys., 2011, 13, 6205–6210 RSC
.
- V. Subramanian, E. E. Wolf and P. V. Kamat, Catalysis with TiO2/Gold Nanocomposites. Effect of Metal Particle Size on the Fermi Level Equilibration, J. Am. Chem. Soc., 2004, 126, 4943–4950 CrossRef CAS PubMed
.
- Y. M. Huang, Q.-L. Ma and B.-G. Zhai, Core-Shell Zn/ZnO Structures with Improved Photocatalytic Properties Synthesized by Aqueous Solution Metod, Funct. Mater. Lett., 2013, 06, 1350058 CrossRef
.
- V. L. Prasanna and V. Rajagopalan, A New Synergetic Nanocomposite for Dye Degradation in Dark and Light, Sci. Rep., 2016, 6, 38606 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, supporting figures, supporting table and computational details. See DOI: 10.1039/c8en01095a |
| This journal is © The Royal Society of Chemistry 2019 |