Titanium dioxide nanofibers induce angiogenic markers and genomic instability in lung cells leading to a highly dedifferentiated and fibrotic tumor formation in a xenograft model
Estefany I.
Medina-Reyes†
 a,
Norma L.
Delgado-Buenrostro
a,
Norma L.
Delgado-Buenrostro
 a,
Alejandro
Déciga-Alcaraz†
a,
Alejandro
Déciga-Alcaraz†
 a,
Verónica
Freyre-Fonseca
a,
Verónica
Freyre-Fonseca
 a,
José O.
Flores-Flores
b,
Rogelio
Hernández-Pando
c,
Jorge
Barrios-Payán
c,
Julio C.
Carrero
d,
Yesennia
Sánchez-Pérez
a,
José O.
Flores-Flores
b,
Rogelio
Hernández-Pando
c,
Jorge
Barrios-Payán
c,
Julio C.
Carrero
d,
Yesennia
Sánchez-Pérez
 e,
Claudia M.
García-Cuéllar
e,
Claudia M.
García-Cuéllar
 e,
Felipe
Vaca-Paniagua
e,
Felipe
Vaca-Paniagua
 aef and
Yolanda I.
Chirino
aef and
Yolanda I.
Chirino
 *a
*a
aLaboratorio de Carcinogénesis y Toxicología, Unidad de Biomedicina, Facultad de Estudios Superiores, Universidad Nacional Autónoma de México, Av. De los Barrios 1, Col. Los Reyes Iztacala, Tlalnepantla, CP 54059, Estado de México, Mexico. E-mail: irasemachirino@gmail.com; Tel: +52 55 56231333
bInstituto de Ciencias Aplicadas y Tecnología, Universidad Nacional Autónoma de México, Circuito Exterior S/N, Ciudad Universitaria, AP 70-186, CP 04510, Ciudad de México, Mexico
cSección de Patología Experimental, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Vasco de Quiroga 15, Colonia Sección XVI, Tlalpan, CP 14000, Ciudad de México, Mexico
dDepartamento de Inmunología, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, Ciudad Universitaria, CP 04510 Ciudad de México, Mexico
eSubdirección de Investigación Básica, Instituto Nacional de Cancerología, San Fernando No. 22, Tlalpan, CP 14080 Ciudad de México, Mexico
fLaboratorio Nacional en Salud: Diagnóstico Molecular y Efecto Ambiental en Enfermedades Crónico-Degenerativas, Facultad de Estudios Superiores Iztacala, Universidad Nacional Autónoma de México, Av. De los Barrios 1, Col. Los Reyes Iztacala, Tlalnepantla, CP 54059, Estado de México, Mexico
First published on 26th November 2018
Abstract
The synthesis of novel nanofibers has increased due to their broad spectrum of applications which have raised also the concern of toxicity mediated by inhalation. Titanium dioxide (TiO2), classified as a possible carcinogen to humans by the International Agency for Research on Cancer regardless of size and shape, is being manufactured as nanofibers for waste water cleaning, extraction of enhanced miRNAs, glucose quantification and other devices. Toxicological studies of TiO2 nanofibers have shown their capability to induce sustained inflammation, frustrated phagocytosis, and lysosomal disruption, but the capability to acquire or enhance aggressive characteristics, including angiogenesis, fibrosis or epithelial mesenchymal transition (EMT) has not been investigated. In this study, we synthetized TiO2 nanofibers (anatase phase; 61.5 ± 4.9 nm width and 3.1 ± 0.2 μm length) to expose monolayers of lung epithelial cells (1 and 10 μg cm−2) for 7 days, and angiogenesis, fibrosis, EMT markers, genomic instability and cisplatin sensitivity were measured. Then, those cells were harvested and injected subcutaneously in a xenograft mouse model for tumor development. After 11 weeks, the same markers were measured in the tumors. The monolayers exposed to TiO2 nanofibers induced angiogenic, fibrotic and EMT markers, genomic instability and loss of cisplatin sensitivity. The tumors developed from exposed cells to TiO2 nanofibers were also positive for the same markers, and moreover, dedifferentiation, the remarkable presence of erythrocytes and loss of cisplatin sensitivity were higher, which suggests that TiO2 nanofibers enhance the aggressive tumor phenotype in lung epithelial cells.
Environmental significanceTitanium dioxide manufacture was estimated to be 6.6 million tons between 2008 and 2012, and some of this production was used for nanofiber synthesis with the purpose of developing water and air pollutant removal systems, biosensors and devices for molecular biology. However, the concern of nanofiber inhalation is increasing in occupational settings due to the evidence of shape toxicity. Beyond the cytotoxicity induced by nanofiber exposure, promotion of an aggressive phenotype has been less investigated. The originality of this study is based on the demonstration that lung adenocarcinoma cells exposed to TiO2 nanofibers enhanced tumor characteristics including angiogenic markers and genomic instability, and these cells can even acquire a more aggressive phenotype when grown in a xenograft nude mouse model. |
1. Introduction
The production of engineered nanomaterials has dramatically increased in the last 15 years. Nowadays, some databases such as the Nanowerk Nanomaterial Database™ contain about 4000 different nanomaterials including nanotubes, fullerenes, graphene, quantum dots, nanoparticles, nanowires and nanofibers.1 The application of these nanomaterials is extended to medicine, electronics, and agriculture, among others. However, the wide usage of nanomaterials for nanotechnology development has raised the concern of adverse effects to human health specifically for those that are massively produced. For instance, the manufacture of titanium dioxide (TiO2), one of the most used pigments, had an estimated production of 6.6 million tons between 2008 and 2012.2 In the last decade, nanosized TiO2 reached production of about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ton per year3 raising the concern of adverse effects on personnel in occupational settings who inhale nanosized particles. To some extent, the concern comes from the International Agency for Research on Cancer classification of TiO2 in the group 2B as a possible carcinogen to humans4 and from the National Institute for Occupational Safety and Health (USA), which classified TiO2 as a potential occupational carcinogen. These classifications have been done regardless of the shape but in the last decade, TiO2 production has been extended from spherical or amorphous shapes to more fibrous forms, which offer different uses, better properties for some purposes including higher photocatalytic activity efficiency,5 improvements in analyzing organochlorine pesticides from rain and lakes in agriculture,6 biosensors for glucose detection7 and drug delivery systems8 in biomedicine and for isolation of miRNAs as a molecular biology tool.9 The biomedical role of TiO2 nanofibers is attributed to some effects in biological systems, for example, their capability to induce cell adherence, proliferation and cell growth helpful in tissue regeneration10 and also, the capability to promote angiogenesis which is essential for successful osseointegration onto implanted materials.11 However, other properties that make TiO2 nanofibers promising for the development of detection tools in biomedicine is the stable capability of TiO2 to interact with the RNA phosphate backbone.12 Indeed, the amount of TiO2 nanofiber research on device development is higher compared with that on side effects associated with inhalation during manufacture, but fibers exhibit important toxicity according to in vitro and in vivo studies.13,14 For instance, tin oxide nanofibers induced inflammation without signs of cell membrane damage.15 Ceramic fibers, which have been classified in the group 2B by the IARC, exhibit cytotoxic and genotoxic effects in lung epithelial cells.16 Asbestos and some other fiber-like materials such as multi-walled carbon nanotubes are able to induce positive markers for epithelial mesenchymal transition (EMT) in lung epithelial cells.17,18
000 ton per year3 raising the concern of adverse effects on personnel in occupational settings who inhale nanosized particles. To some extent, the concern comes from the International Agency for Research on Cancer classification of TiO2 in the group 2B as a possible carcinogen to humans4 and from the National Institute for Occupational Safety and Health (USA), which classified TiO2 as a potential occupational carcinogen. These classifications have been done regardless of the shape but in the last decade, TiO2 production has been extended from spherical or amorphous shapes to more fibrous forms, which offer different uses, better properties for some purposes including higher photocatalytic activity efficiency,5 improvements in analyzing organochlorine pesticides from rain and lakes in agriculture,6 biosensors for glucose detection7 and drug delivery systems8 in biomedicine and for isolation of miRNAs as a molecular biology tool.9 The biomedical role of TiO2 nanofibers is attributed to some effects in biological systems, for example, their capability to induce cell adherence, proliferation and cell growth helpful in tissue regeneration10 and also, the capability to promote angiogenesis which is essential for successful osseointegration onto implanted materials.11 However, other properties that make TiO2 nanofibers promising for the development of detection tools in biomedicine is the stable capability of TiO2 to interact with the RNA phosphate backbone.12 Indeed, the amount of TiO2 nanofiber research on device development is higher compared with that on side effects associated with inhalation during manufacture, but fibers exhibit important toxicity according to in vitro and in vivo studies.13,14 For instance, tin oxide nanofibers induced inflammation without signs of cell membrane damage.15 Ceramic fibers, which have been classified in the group 2B by the IARC, exhibit cytotoxic and genotoxic effects in lung epithelial cells.16 Asbestos and some other fiber-like materials such as multi-walled carbon nanotubes are able to induce positive markers for epithelial mesenchymal transition (EMT) in lung epithelial cells.17,18
Regarding to TiO2 nanofibers, their exposure induces loss of membrane integrity in lung epithelial cells19 while their pharyngeal aspiration caused pulmonary inflammation in a dose and time-dependent manner.20 Lung epithelial cells exposed to TiO2 nanofibers for 7 days not only survived, but also gained invasive and proliferative potential after 5 days of being seeded into a chorioallantoic membrane.21 It is important to highlight that the impact of nanofibers and other nanoparticles in epithelial cells is relevant because 90% of the tumors develop from these types of cells. It has been demonstrated that lung epithelial cells are able to develop tumors in a xenograft mouse model and we took advantage of this model to study tumor growth and characteristics from those tumor cells.22,23 Indeed, this model allows in vitro treatments of cells that later can be analyzed after tumor formation in vivo. Then, we hypothesized that exposure of lung epithelial cells to TiO2 nanofibers could induce a more aggressive tumor phenotype when cells are grown in the xenograft model. To test our hypothesis, we aimed to synthetize TiO2 nanofibers and expose them to lung epithelial cells for 7 days to be subcutaneously injected into a xenograft mouse model. Angiogenesis, fibrosis, EMT markers, genomic instability, and cisplatin sensitivity were evaluated in the cell cultures after 7 days of continuous exposure and in excised tumors after 11 weeks.
2. Methods
2.1. Synthesis of TiO2 nanofibers
To synthesize TiO2 nanofibers, 0.5 g of anatase TiO2 nanoparticles (Aldrich, 637254) was suspended in 35 mL of 10 M NaOH solution. The solution was ultrasonicated for 10 min, then poured into a steel-container and sealed. For the hydrothermal treatment, the container was heated at 200 °C for 24 h. Wet TiO2 obtained after the hydrothermal treatment was repeatedly washed with distilled water and 0.1 M HCl until pH 7 was reached, and centrifuged between each wash at 3.5 × g for 15 min. Calcination of the TiO2 pellet was reached at 700 °C for 30 min at a ramp rate of 1 °C min−1.132.2. Characterization of TiO2 nanofibers
TiO2 nanofiber size and morphology were observed by scanning electron microscopy (SEM; JEOL 5800-LV, Japan) at 5000× and 15 kV. The size and morphology of the agglomerates formed in F12K cell culture medium supplemented with 10% fetal bovine serum (FBS) were analyzed by transmission electron microscopy (TEM; JEOL JEM 1010, Japan) and observed at 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× 60 kV. Raman spectra of TiO2 nanofibers were measured in the 300–2000 cm−1 spectral region with a confocal Raman microscope WITec alpha300 R system at room temperature. The Raman laser excitation wavelength is 532 nm and the spectral resolution of the spectrometer is 4–5 cm−1. A Nikon 10 objective was used to focus the laser on a 1
000× 60 kV. Raman spectra of TiO2 nanofibers were measured in the 300–2000 cm−1 spectral region with a confocal Raman microscope WITec alpha300 R system at room temperature. The Raman laser excitation wavelength is 532 nm and the spectral resolution of the spectrometer is 4–5 cm−1. A Nikon 10 objective was used to focus the laser on a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mm spot. The zeta potential of the TiO2 nanofibers suspended in F12K cell culture medium supplemented with 10% FBS was measured using a Zeta Plus meter Brookehaven (USA) and the agglomerate size was measured by dynamic light scattering (DLS) (NS-Zeta sizer Malvern, UK). The measurements of the zeta potential and size of the agglomerates were carried out at 23 ± 1 °C and at pH 7.4. The length and width of the fibers were measured from 5 SEM images and analyzed using ImageJ 1.43 software (National Institutes of Health, USA).24
5 mm spot. The zeta potential of the TiO2 nanofibers suspended in F12K cell culture medium supplemented with 10% FBS was measured using a Zeta Plus meter Brookehaven (USA) and the agglomerate size was measured by dynamic light scattering (DLS) (NS-Zeta sizer Malvern, UK). The measurements of the zeta potential and size of the agglomerates were carried out at 23 ± 1 °C and at pH 7.4. The length and width of the fibers were measured from 5 SEM images and analyzed using ImageJ 1.43 software (National Institutes of Health, USA).24
2.3. In vitro model
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× and 60 kV. To visualize cell morphology, the cell cultures were fixed in paraformaldehyde 4% and stained using hematoxylin for 30 seconds and eosin for 5 minutes. The samples were observed by optical microscopy (Confocal Microscope Leica TCS SP8 X, Germany) at a magnification of 40×.
000× and 60 kV. To visualize cell morphology, the cell cultures were fixed in paraformaldehyde 4% and stained using hematoxylin for 30 seconds and eosin for 5 minutes. The samples were observed by optical microscopy (Confocal Microscope Leica TCS SP8 X, Germany) at a magnification of 40×.
For RNA Ki67 detection, which is a proliferation marker, after 7 days of TiO2 nanofiber exposure, cell monolayer cultures were washed with PBS and 1 mL Trizol was added to each sample for RNA extraction. The integrity and purity of each sample was verified by agarose electrophoresis. A reverse transcriptase was used to synthetize complementary DNA (cDNA) from the RNA obtained from each (RT-PCR method) sample, and Ki67 expression and analysis were performed using Image J 1.43 software. The Ki67 protein level was detected by immunocytochemistry using an anti-Ki67 antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 dilution; BioLegend, 652402) and diaminobenzidine solution as a substrate for secondary antibody detection and counterstained with hematoxylin. A semi-quantification was performed using a confocal microscope Leica TCS SP8 X, and at least three independent experiments were done with negative controls. Data are presented as mean ± standard error. Ki67 forward primer GCCTGCTCGACCCTACAGA; reverse primer GCTTGTCAACTGCGGTTGC. Actin gene expression was used as the control for RT-PCR.
500 dilution; BioLegend, 652402) and diaminobenzidine solution as a substrate for secondary antibody detection and counterstained with hematoxylin. A semi-quantification was performed using a confocal microscope Leica TCS SP8 X, and at least three independent experiments were done with negative controls. Data are presented as mean ± standard error. Ki67 forward primer GCCTGCTCGACCCTACAGA; reverse primer GCTTGTCAACTGCGGTTGC. Actin gene expression was used as the control for RT-PCR.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 dilution, Santa Cruz 146; Santa Cruz H206) with TRITC as the secondary antibody (dilution 1
300 dilution, Santa Cruz 146; Santa Cruz H206) with TRITC as the secondary antibody (dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 Jackson 111-025-003), and co-localization of HIF-1α in the nucleus, which was stained with Hoechst (Thermo Scientific, 62249). Co-localization was evaluated using Pearson's correlation using the confocal microscope Leica TCS SP8 X software. Images from each treatment were obtained by confocal microscopy (Confocal Microscope Leica TCS SP8 X, Germany). N-Cadherin expression was determined by immunocytochemistry (1
600 Jackson 111-025-003), and co-localization of HIF-1α in the nucleus, which was stained with Hoechst (Thermo Scientific, 62249). Co-localization was evaluated using Pearson's correlation using the confocal microscope Leica TCS SP8 X software. Images from each treatment were obtained by confocal microscopy (Confocal Microscope Leica TCS SP8 X, Germany). N-Cadherin expression was determined by immunocytochemistry (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 dilution; BioLegend, 652402) and protein detection was performed using diaminobenzidine solution and slides counterstained with hematoxylin. Three independent experiments with negative controls were performed. Arbitrary units of the fluorescence of three independent mice tumors were analyzed using the confocal microscope Leica TCS SP8 X software and data are presented as mean ± standard error. HIF-1α forward primer TTCCCGACTAGGCCCATTC; reverse primer CAGGTATTCAAGGTCCCATTTCA. VEGF forward primer GGCTGGCAACATAACAGAGAA; reverse primer CCCCACATCTATACACACCTCC.
500 dilution; BioLegend, 652402) and protein detection was performed using diaminobenzidine solution and slides counterstained with hematoxylin. Three independent experiments with negative controls were performed. Arbitrary units of the fluorescence of three independent mice tumors were analyzed using the confocal microscope Leica TCS SP8 X software and data are presented as mean ± standard error. HIF-1α forward primer TTCCCGACTAGGCCCATTC; reverse primer CAGGTATTCAAGGTCCCATTTCA. VEGF forward primer GGCTGGCAACATAACAGAGAA; reverse primer CCCCACATCTATACACACCTCC.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 3 min and the supernatant was used for hydroxyproline determination using the Biovision Kit K555. Briefly, a standard curve with known concentrations was obtained and 10 μL of previous hydrolyzed samples were used to determine the hydroxyproline concentration.25,26 Both standard curve and hydrolyzed samples were incubated at 60 °C for 90 min and the absorbance was determined at 560 nm using a microplate reader. Data are presented as mean ± standard error.
000 × g for 3 min and the supernatant was used for hydroxyproline determination using the Biovision Kit K555. Briefly, a standard curve with known concentrations was obtained and 10 μL of previous hydrolyzed samples were used to determine the hydroxyproline concentration.25,26 Both standard curve and hydrolyzed samples were incubated at 60 °C for 90 min and the absorbance was determined at 560 nm using a microplate reader. Data are presented as mean ± standard error.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 (Thermo Scientific, 62249) at 37 °C under constant agitation for 1 h. Then, the coverslips from each sample were removed from the plate and mounted on glass slides which were scored using a fluorescence microscope to obtain the frequency of binucleated cells with a micronucleus and 1000 binucleated cells were scored per slide. To obtain the frequency of trinucleated and tetranucleated cells, 1000 binucleated, trinucleated and tetranucleated cells were scored per slide with at least 3 slides per treatment. Data are presented as mean ± standard error.
800 (Thermo Scientific, 62249) at 37 °C under constant agitation for 1 h. Then, the coverslips from each sample were removed from the plate and mounted on glass slides which were scored using a fluorescence microscope to obtain the frequency of binucleated cells with a micronucleus and 1000 binucleated cells were scored per slide. To obtain the frequency of trinucleated and tetranucleated cells, 1000 binucleated, trinucleated and tetranucleated cells were scored per slide with at least 3 slides per treatment. Data are presented as mean ± standard error.
2.4. Xenograft model
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 dilution, Santa Cruz 146) followed by a TRITC-secondary antibody (dilution 1
300 dilution, Santa Cruz 146) followed by a TRITC-secondary antibody (dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 Jackson 111-025-003). Arbitrary units of fluorescence of three independent mice tumors were analyzed using the confocal microscope Leica TCS SP8 X software, and data are presented as mean ± standard error. To detect collagen in tumor sections, Masson trichrome staining was performed. Briefly, the 3 μm sections were immersed in Bouin's Solution at room temperature and in darkness for 72 h. Then, the sections were stained with Weigert's hematoxylin for 10 min, Biebrich scarlet-acid fuchsine for 10 min and phosphomolybdic–phosphotungstic acid followed by aniline blue solution for 10 min. The positive area for connective tissue (blue stained) was semi-quantified with the Image J 1.43 software (National Institutes of Health, USA). To confirm the collagen content, immunocytochemistry was done (1
600 Jackson 111-025-003). Arbitrary units of fluorescence of three independent mice tumors were analyzed using the confocal microscope Leica TCS SP8 X software, and data are presented as mean ± standard error. To detect collagen in tumor sections, Masson trichrome staining was performed. Briefly, the 3 μm sections were immersed in Bouin's Solution at room temperature and in darkness for 72 h. Then, the sections were stained with Weigert's hematoxylin for 10 min, Biebrich scarlet-acid fuchsine for 10 min and phosphomolybdic–phosphotungstic acid followed by aniline blue solution for 10 min. The positive area for connective tissue (blue stained) was semi-quantified with the Image J 1.43 software (National Institutes of Health, USA). To confirm the collagen content, immunocytochemistry was done (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 dilution; BioLegend, 652402). On the other hand, fibrotic markers TGF-β, E-Cadherin and Vimentin were detected in the tumor sections using immunofluorescence (1
500 dilution; BioLegend, 652402). On the other hand, fibrotic markers TGF-β, E-Cadherin and Vimentin were detected in the tumor sections using immunofluorescence (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 dilution; Santa Cruz H206; 1
300 dilution; Santa Cruz H206; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 dilution Biolegend 324102; 1
600 dilution Biolegend 324102; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 dilution Santa Cruz 7558, respectively). Then, TGF-β and E-Cadherin samples were incubated with the secondary TRITC-antibody (dilution 1
500 dilution Santa Cruz 7558, respectively). Then, TGF-β and E-Cadherin samples were incubated with the secondary TRITC-antibody (dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 Jackson 111-025-003) and vimentin samples were incubated with the FITC-antibody (dilution 1
600 Jackson 111-025-003) and vimentin samples were incubated with the FITC-antibody (dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 Jackson 115-095-003). Images at 40× magnification from each treatment were taken using a confocal microscope and semi-quantification with the Leica TCS SP8 software was done (Confocal Microscope Leica TCS SP8 X, Germany). Three independent experiments were done. Negative controls were performed by excluding the primary antibody.
600 Jackson 115-095-003). Images at 40× magnification from each treatment were taken using a confocal microscope and semi-quantification with the Leica TCS SP8 software was done (Confocal Microscope Leica TCS SP8 X, Germany). Three independent experiments were done. Negative controls were performed by excluding the primary antibody.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 events were read. Data are presented as mean ± standard error of at least three independent experiments. For CDDP treatment, cells from monolayer cultures and isolated cells from excised xenograft tumors were exposed to 0, 25, 50, 75 and 100 μM CDDP for 24 h and the half maximal inhibitory concentration (IC50) was determined using the MTT assay. Briefly, the supernatants were discarded from the cell culture and each well was incubated with 100 μL of FBS-free medium containing 0.5 mg mL−1 MTT at 37 °C for 2 h. Formazan crystals were dissolved in isopropyl alcohol and the samples were centrifuged at 126 × g for 5 min to avoid nanoparticles and optical density was measured at 540 nm. Data are presented as mean ± standard error of three independent experiments.
000 events were read. Data are presented as mean ± standard error of at least three independent experiments. For CDDP treatment, cells from monolayer cultures and isolated cells from excised xenograft tumors were exposed to 0, 25, 50, 75 and 100 μM CDDP for 24 h and the half maximal inhibitory concentration (IC50) was determined using the MTT assay. Briefly, the supernatants were discarded from the cell culture and each well was incubated with 100 μL of FBS-free medium containing 0.5 mg mL−1 MTT at 37 °C for 2 h. Formazan crystals were dissolved in isopropyl alcohol and the samples were centrifuged at 126 × g for 5 min to avoid nanoparticles and optical density was measured at 540 nm. Data are presented as mean ± standard error of three independent experiments.
2.5. Statistical analysis
One-way ANOVA was used to analyze differences between treatments followed by a Bonferroni test for differences between means. Two-way ANOVA was used to analyze differences between the in vitro model and xenograft model followed by a Bonferroni test for differences between means. Data were presented as mean ± standard error.3. Results
3.1. TiO2 nanofiber characterization
Shape and primary size of the TiO2 nanofibers were obtained by TEM (Fig. 1A). The TiO2 nanofibers dispersed in cell culture medium supplemented with 10% FBS had a hydrodynamic size of 249.5 ± 12.8 nm (Fig. 1B) and a zeta potential of −27.17 ± 3.72 mV. In addition, to evaluate if the composition of TiO2 nanofibers was preserved after the hydrothermal process, Raman spectroscopy was performed showing the typical pattern of anatase phase absorption (Fig. 1C). The high purity of TiO2 nanofibers is also evident in the Raman spectra, where the typical modes of TiO2 anatase phase at 394, 515, and 638 cm−1 can be clearly observed. It is well known that anatase has the following Raman-active modes: A1g + 2B1g + 3Eg and three infrared active modes, A2u + 2Eu.31 We observed only the main Raman peaks of anatase at 400 cm−1 (B1g), 516 cm−1 (A1g or B1g), and 639 cm−1 (Eg) and of rutile at 449 cm−1 (Eg) and at 612 cm−1 (A1g). This indicates that the unique phase present in the sample is anatase. The TiO2 nanofibers have a 3.1 ± 0.2 μm length and a 61.5 ± 4.9 nm width (Fig. 1D).3.2. TiO2 nanofibers were incorporated by cells exposed in monolayer cultures
After 7 days of continuous TiO2 exposure, adenocarcinoma epithelial cells internalized the nanofibers and those were located in vesicles together with some lamellar bodies in the cells exposed to 1 or 10 μg cm−2 (Fig. 2A). Cell morphology remained without remarkable alterations after TiO2 nanofiber exposure examined by hematoxylin/eosin staining or differential interference contrast microscopy; however, both methods allowed the observation of TiO2 nanofiber deposits in the cytoplasm and around the nucleus (Fig. 2A–C). TiO2 nanofibers were found closer to lamellar bodies, which is consistent with some other NPs, including gold nanoparticles and silica-coated superparamagnetic iron oxide nanoparticles that have also been located surrounding lamellar bodies.32,33 The relevance of this finding is based on the fact that using in vivo models, it has been determined that disturbances in the metabolism of the surfactant released by lamellar bodies is implicated in the pathological process related to fibrosis and EMT.34–363.3. TiO2 nanofibers induced slight cytotoxicity without cell proliferation in monolayer cultures
After TiO2 nanofiber exposure, the cytotoxicity in adenocarcinoma epithelial cells evidenced by LDH release showed an increment of 22 ± 2.9 and 48 ± 7.9% in the supernatant of the 1 or 10 μg cm−2 treatments, respectively (Fig. 3A). This result is in line with a previous study in which it was demonstrated that exposure to TiO2 fibers induces LDH release in alveolar macrophages.37 Ki67 gene and protein expression remained without changes (Fig. 3B and C). Indeed, cells exposed to 10 μg cm−2 TiO2 nanofibers showed 17.8% less Ki67 protein expression compared with control cells (Fig. 3D). This could be attributed to the fact that nanofibers induce cell damage as was demonstrated by LDH release and the cells had decreased proliferative capability. Beyond the toxicity, the question is what type of alterations on the surviving cells after TiO2 NPs exposure can be preserved. In the following sections, we demonstrate that in spite of cytotoxicity, exposed cells are positive for markers related to pro-carcinogenic processes.3.4. TiO2 nanofibers induced upregulation of angiogenesis markers in monolayer cultures
The expression of HIF-1α in untreated cells was perinuclear and in contrast, cells exposed to 1 μg cm−2 TiO2 nanofibers showed a nuclear HIF-1α localization that was more evident in cells exposed to 10 μg cm−2 (Fig. 4A). A Pearson's correlation between HIF-1α and the nuclear colocalization confirmed this observation and showed that the control cells had a −0.3 ± 0.04 value while the cells exposed to 1 or 10 μg cm−2 TiO2 nanofibers had −0.15 ± 0.03 and 0.05 ± 0.02, respectively (0 < r ≤ 1 and −1 < r ≤ 0, values indicate positive or negative correlation, respectively; Fig. 4C). Protein immunodetection of HIF-1α in the control cells resulted to 883.8 ± 83.2 AU, and an increase was detected in cells exposed to 1 or 10 μg cm−2 TiO2 nanofibers, which was 1505 ± 57 AU and 1456 ± 50.9 AU, respectively (Fig. 4B). HIF-1α gene expression increased 33% and 73% after 1 μg cm−2 and 10 μg cm−2 TiO2 nanofiber treatment, respectively (Fig. 4F and G). VEGF gene expression increased 25% and 37% after 1 μg cm−2 or 10 μg cm−2 TiO2 nanofiber treatment (Fig. 4D and E). Additionally, VEGF increased from 0.80 ± 0.04 AU to 1.0 ± 0.09 and 1.14 ± 0.13 fold of change after 1 μg cm−2 or 10 μg cm−2 TiO2 nanofiber exposure, respectively (Fig. 4D and E).3.5. TiO2 nanofibers induced upregulation of pro-fibrotic markers in monolayer cultures
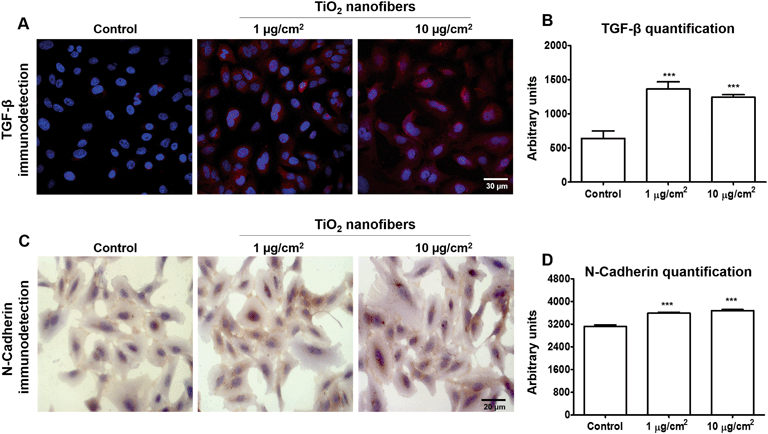
Deregulation of TGF-β has been related to cancer through modulation of fibrosis, angiogenesis and EMT,38–40 then we decided to measure the expression of this factor as a possible link between angiogenesis, fibrosis and EMT markers found upregulated in cell cultures. Here, we found that cells exposed to both concentrations of TiO2 nanofibers showed an upregulation in TGF-β expression in the cytoplasm (Fig. 5A). A quantification of this expression showed an increase of 2.13-fold and 1.95-fold in cells exposed to 1 μg cm−2 or 10 μg cm−2 TiO2 nanofibers, respectively (Fig. 5B). N-Cadherin expression also had a slight increase in expression in the cytoplasm (Fig. 5C) of 15% and 18% of protein after 1 μg cm−2 or 10 μg cm−2 TiO2 nanofiber treatments, respectively (Fig. 5D). Hydroxyproline, an amino acid found only in collagen and elastin in mammals and used as a pro-fibrotic marker, was found increased from 2.3 ± 0.6 μM in control cells to 5.5 ± 1.1 μM and 9.9 ± 1.3 μM in exposed cells to 1 or 10 μg cm−2, respectively. This increase corresponds to 237% and 423% more hydroxyproline in exposed cells compared with control cells, indicating a fibrotic response after TiO2 nanofiber exposure (Fig. 6).3.6. TiO2 nanofibers induced genomic instability in monolayer cultures
Treatment with 10 μg cm−2 TiO2 nanofibers increased genomic instability measured as micronuclei formation from 44 ± 1.7 per 1000 binucleated cells in the control cells to 77 ± 6.5 per 1000 binucleated cells in the cells exposed to 10 μg cm−2 TiO2 nanofibers (Fig. 7A); no significant increase in binucleated cells with micronuclei was observed in cells exposed to 1 μg cm−2 (Fig. 7B) but multinucleated cells increased with both TiO2 nanofibers treatments (Fig. 7C) and increased from 90 ± 10 trinucleated cells per 1000 cells in control cells to 133 ± 3.6 and 147 ± 12 in cells exposed to 1 and 10 μg cm−2 TiO2 nanofibers, respectively (Fig. 7D). Tetranucleated cells only increased in the cells treated with 10 μg cm−2 TiO2 nanofibers from 50 ± 7 tetranucleated cells per 1000 cells to 74 ± 6.5 tetranucleated cells per 1000 cells (Fig. 7E).3.7. Monolayer cultures exposed to TiO2 nanofibers induced tumors in the xenograft model with irregular shapes, dedifferentiation, increased erythrocyte infiltration and high HIF-1α expression
Lung epithelial cells unexposed and exposed to 1 or 10 μg cm−2 TiO2 nanofibers for 7 days were used for induction of the ectopic xenograft model, and tumors were dissected after 11 weeks. During this period, food intake (Fig. 8A), water consumption (Fig. 8B) and body weight (Fig. 8C) of the mice with tumors remained without changes. There was no statistical difference between the volume of tumors developed for 11 weeks from the control cells and those developed from cell cultures treated with 1 μg cm−2 or 10 μg cm−2 TiO2 nanofibers (Fig. 8D). However, the excised tumors from control cells showed a three-dimensional spherical structure with an average diameter of 1.2 cm, while the excised tumors derived from the exposed cells to 1 μg cm−2 or 10 μg cm−2 TiO2 nanofibers showed a three-dimensional irregular structure (Fig. 9A and B), which can be also seen in binary images of the tumors (Fig. 9C). The histological study of control tumors derived from unexposed cells showed moderately differentiated adenocarcinoma constituted by mantles of polygonal neoplastic cells with occasional well-formed glandular structures (Fig. 10A). However, while tumors derived from cells exposed to 1 μg cm−2 TiO2 nanofibers showed a lesser degree of differentiation, manifested by mantles of large polygonal cells, many of them with big atypical hyperchromatic nuclei alternated with areas of smaller cuboidal cells (Fig. 10A). An even lesser degree of differentiation was observed in tumors constituted by cells exposed to 10 μg cm−2 TiO2 nanofibers, which showed focal areas of fusiform sarcomatoid cells (Fig. 10A). The histological analysis of excised tumors showed an adenocarcinoma pattern with prominent infiltration of erythrocytes (Fig. 10A). Differentiated tumors have a tendency to spread faster than well-differentiated tumors. Then, we observed an important presence of erythrocytes, and a quantification was performed. Results showed 14 ± 0.7 erythrocytes per field while 56 ± 0.9 erythrocytes and 36 ± 1.6 erythrocytes per field were counted in tumors derived from cells exposed to 1 μg cm−2 or 10 μg cm−2 TiO2 nanofibers, respectively (Fig. 10B). Since HIF-1α upregulation plays a role in the de novo vessel formation from pre-existing blood vessels which leads to erythrocyte supply, we determined the expression of HIF-1α in the tumors. HIF-1α was predominantly found in the cytoplasm (Fig. 10C) and had a 2.5 and 6.2-fold increase in expression in tumors derived from cells exposed to 1 μg cm−2 TiO2 nanofibers and 10 μg cm−2 TiO2 nanofibers, respectively (Fig. 10D).3.8. Monolayer cultures exposed to TiO2 nanofibers induced proliferative and fibrotic tumors in the xenograft model
Proliferation and apoptosis were evaluated in tumors derived from exposed cells to TiO2 nanofibers. The Ki67 proliferation marker increased in a similar pattern in both treatments (Fig. 11A) and the quantification of immunostaining showed an increment from 1333 ± 20 AU in the control tumors to 2074 ± 131 and 2257 ± 54 AU in the 1 and 10 μg cm−2 TiO2 nanofiber derived tumors, respectively (Fig. 11B). The detection of caspase 3, an apoptosis marker, decreased in the tumors derived from TiO2 nanofiber exposed cells (Fig. 11C) and its quantification showed values of 1829 ± 67 AU for tumors derived from control cells and 1552 ± 56 AU and 1488 ± 50 AU, respectively for tumors derived from 1 and 10 μg cm−2, (Fig. 11D).Fibrotic tissue was detected in all tumors by Masson's trichrome staining in the periphery of the tumors and as collagen fibers in the central tumor areas (Fig. 12A). The area of positive staining increased from 922 ± 40 μm2 per field in the control tumors to 3974 ± 281 μm2 per field and 7330 ± 1083 μm2 per field in the tumors derived from cells exposed to 1 and 10 μg cm−2 TiO2 nanofibers (Fig. 12B). Besides, collagen I immunostaining was performed in all tumors (Fig. 12C) and positive staining was quantified. Tumors derived from cells exposed to TiO2 nanofibers had an increase from 1110 ± 39 AU in the control tumors to 2161 ± 75 AU and 1969 ± 43 AU in tumors derived from cells exposed to 1 μg cm−2 or 10 μg cm−2 TiO2 nanofibers, respectively (Fig. 12D).
3.9. Monolayer cultures exposed to TiO2 nanofibers induced tumors positive for EMT markers in the xenograft model
Based on the histology alterations and the collagen type I upregulation, we investigated the expression of TGF-β, E-cadherin, vimentin and N-cadherin in tumors which are the known mediators of EMT. TGF-β expression in the control tumors was 22 ± 0.8 AU and it increased to 49 ± 2.3 AU and 46 ± 1.7 AU in the tumors derived from cells exposed to 1 μg cm−2 or 10 μg cm−2 TiO2 nanofibers, respectively (Fig. 13A and B). In contrast, a decrease in the degree of immunostaining of E-cadherin was found from 549 ± 14 AU in the control tumors to 427 ± 20 AU and 347 ± 16 AU in the tumors derived from exposed cells to 1 and 10 μg cm−2 TiO2 nanofibers (Fig. 13C and D). In this regard, loss of E-cadherin expression could be related to the matrix metalloproteinases upregulation, which enhance the degradation of the extracellular matrix but could also disrupt the interaction between E-cadherin and beta-catenin, and the deregulation between these proteins has been extensively associated with cancer development.Then, we detected that vimentin remained without changes among all groups of tumors (Fig. 13E and F), while immunodetection of N-cadherin increased from 1330 ± 45 AU in the control tumors to 2290 ± 116 AU and 2203 ± 57 AU in the tumors derived from the 1 or 10 μg cm−2 TiO2 nanofiber treatments, respectively (Fig. 13G and H). N-Cadherin upregulation not only correlates with EMT but has also been associated with more invasive tumors.
3.10. Monolayer cultures exposed to TiO2 nanofibers had no effect on cell cycle distribution but decreased cisplatin sensitivity and is enhanced in the tumors
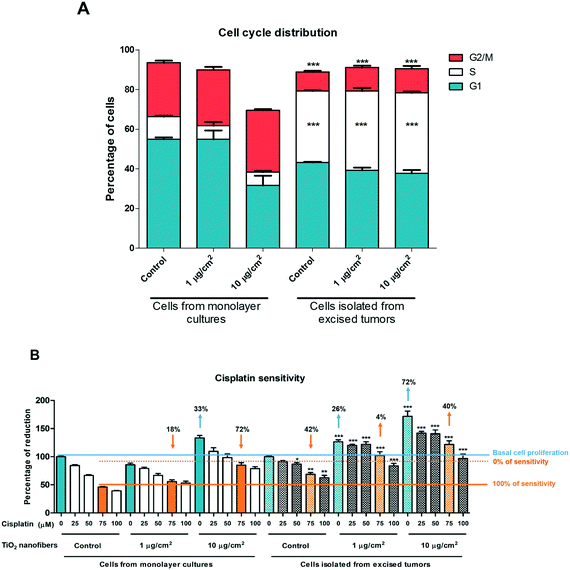
We compared the cell cycle distribution and the susceptibility to cisplatin treatment between the cells derived from the monolayers and the cells derived from tumors (Fig. 14). No alterations in the cell cycle distribution were found between treatments in the cell culture for 7 days and between cells derived from tumors after 11 weeks of growth. However, the S phase in the cell monolayer culture had a cell distribution of 11 ± 0.3% and after tumor development, those cells increased the S phase to 36 ± 0.4%. Cell monolayers treated with 1 μg cm−2 of TiO2 nanofibers had an S phase cell distribution of 6.8 ± 1.8% while those cells after tumor development increased to 40 ± 1.5%. Similar values were obtained for cell monolayers treated with 10 μg cm−2 TiO2 nanofibers, which had a cell distribution of 6 ± 0.6% in the S phase while the tumors had 40 ± 0.6%. The G2/M phase decreased in the cells derived from tumors while the monolayers had higher cell population in this phase. The G2/M phase had 27 ± 1.0% of cells derived from monolayers, and this population decreased to 9 ± 0.5% in the control tumor cells. Similar percentages in the G2/M phase were observed after TiO2 nanofiber exposure in both concentrations. The percentage of exposed cells to 1 and 10 μg cm−2 TiO2 nanofibers in the cell monolayer culture in the G2/M phase was 28 ± 1.4 and 31 ± 0.5%, while the cells from the tumors had 11 ± 0.5% and 12 ± 1.4% (Fig. 14A). Together, we determined that exposure to TiO2 nanofibers had no influence on cell cycle distribution, but tumor cells in the xenograft model had an upregulation in the S phase cycle. Then those cells were treated with cisplatin for 24 h in order to explore if resistance could be acquired and MTT reduction, a common method for cell viability, was performed. Control cell monolayers showed a decrease of 50% of MTT reduction after 75 μM cisplatin treatment and exposed cell monolayers to 1 and 10 μg cm−2 TiO2 nanofibers showed a decrease of 18% and 72% on cisplatin sensitivity suggesting that TiO2 nanofibers exposure promoted the reduction of cisplatin sensitivity (Fig. 14B). The 10 μg cm−2 TiO2 nanofibers treatment in cell monolayers increased the 33% MTT reduction compared with control cell monolayer cultures. Control cells isolated from tumors and treated with cisplatin decreased 42% cisplatin sensitivity, suggesting that the sole tumor formation in the xenograft model promoted cisplatin resistance (Fig. 14B). Cells exposed to TiO2 nanofibers and growth as a tumor in the xenograft model showed higher cisplatin resistance. Precisely, 1 and 10 μg cm−2 TiO2 nanofibers from these tumors not only loss the cisplatin sensitivity but also had a 4% and 40% of cell viability increased (Fig. 14B).4. Discussion
According to the International Organization for Standardization (SO/TS 27687:2008), the material synthetized for this study falls in the nanofiber category which establishes that nano-objects have two similar external dimensions in the nanoscale and the third dimension significantly larger. In this study we used the hydrothermal method for TiO2 nanofiber synthesis, which is until now one of the suitable methods along with electrospinning, and perhaps in the coming near future some other methods such as green protocols emerge. These green protocols besides NP extraction from plants might offer biological activities including cytotoxicity against cancer cells,41 antibacterial properties42 or organic pollutant decomposition.43In the last decade, an enormous effort has been placed in the development of predictive toxicity based on the physicochemical properties of engineered nanomaterials and until now, we have not succeeded entirely, but we have determined that NPs shaped as fibers exhibit high toxicity.13,19,20 The toxicity of nanofibers has been linked to cellular damage through frustrated phagocytosis,19 inflammation13,44 and, oxidative stress45 in several experimental models but the route of exposure and cell type must also be considered. For instance, limited toxicity has been reported in liver from animals treated by oral gavage with nanofibers at low-dose and/or acute exposure46 while the lungs are the main target for toxicity. In addition, even in lung tissue, epithelial cells showed higher toxicity than macrophages.19 This highlight the relevance of studying lung epithelial cells because they could be more susceptible to TiO2 nanofiber exposure than other cell types, and here in our study, we have found genomic instability, which is a severe threat to DNA integrity. We suggest that this effect might be associated with oxidative stress and genotoxicity and possibly could also be linked to inflammatory response after nanoparticle uptake.47–49 In addition, we also found an increase in HIF-1α, VEGF, TGF-b, and N-cadherin expression in cell monolayers exposed to TiO2 nanofibers. According to the literature, all these cellular events could lead to a tumor with an EMT-like phenotype, specifically in epithelial cells. In addition, the increase in hydroxyproline quantification in cell monolayers can be associated with the augmented amount of collagen observed in tumors which might be driven by activation of the TGF-β pathway,25,50 where expression was upregulated in monolayers and tumors in our study. This is in line with previous literature that has been reported that other types of nanoparticles, such as cerium oxide induces fibrosis and upregulates soluble collagen detection in bronchoalveolar lavage fluid associated with colocalization of TGF-β with alpha-smooth muscle actin.18 In addition, TGF-β has been linked to fibrosis in other in vivo models including fibrosis induced by cerium exposure in rats.36 In addition, beyond toxic effects, fibrotic potential is one of the most significant concerns based on the fact that sustained fibrosis could lead to tumor formation and lung diseases.51,52 We speculate that pro-fibrotic effect could be attributed to activation of NLRP3 inflammasome, since other nanoparticles such as zinc oxide nanoparticles, multiwall carbon nanotubes or TiO2 nanospheres can activate this inflammatory pathway.53 Sustained inflammasome activation as well as interstitial fibrosis was confirmed in exposed mice to 30 μg of TiO2 nanofibers even after 112 days of instillation and approximately 20% of TiO2 nanofibers were retained in the terminal bronchiole near the smallest lymphatic capillaries in the lungs, and this phenomenon was not observed in exposed mice to 30 μg of TiO2 nanospheres.20 Moreover, TiO2 nanofibers might induce toxic effects similar to multiwalled carbon nanotubes including frustrated phagocytosis, lysosomal disruption, and impaired clearance,13,54 and also share some similarities with asbestos (crocidolite).19 Indeed, the toxicity of TiO2 nanofibers also shares some cellular mechanisms with asbestos (crocidolite), for instance, both induce a significant decrease in the trans-epithelial electrical resistance of airway cell monolayers, cell shape perturbation with the longest fibers and incomplete phagocytosis; besides, TiO2 nanofibers induced higher hemolytic activity than crocidolite.19 Additionally, mesothelioma induced by asbestos is attributed to the chronic activation of inflammasome, specifically NLP3 which could also be activated chronically by TiO2 nanofibers.13,55 Overall, we found some differences between the in vitro and the xenograft model, which could be partially explained by differences in spatial interactions on cell organization. Lung epithelial cells cultured as monolayers might have less cell–cell contact leading to more contact with TiO2 nanofibers and showed less proliferative capacity. On the contrary, tumors isolated from the xenograft model showed an uneven proliferation rate that is associated with malignancy.56 For instance, an irregular tumor shape was described in 66.7% of malignant breast phyllodes tumors and from them, 90.9% had low malignancy potential and 25% were benign tumors.56 Analysis on meningioma also showed that 90% of malignant meningioma tumors had irregular shapes.56 In spite of increased angiogenesis and proliferation markers, tumors derived from exposed cells to TiO2 nanofibers had higher necrosis than tumors derived from unexposed cells and one explanation might be an insufficient energy supply for tumor development.55 The cells that survive necrosis during tumor development are considered to be a cell population that is more prone to radiation and chemotherapy resistance.57 The energy supply decrease in tumors causes hypoxia, which explains the increase in markers such as HIF-1α and necrosis in some areas of the tumor. This positive relation between hypoxia and necrosis is commonly observed in colon, breast and lung cancer.58 In summary, we found that 7 days of TiO2 nanofiber exposure in lung epithelial cell cultures promoted dedifferentiation, angiogenesis, fibrosis and EMT and those characteristics were not only preserved in the tumors dissected from the xenograft model after 11 weeks of growth but also the tumors gained some other features including higher vascularity with more erythrocytes which is a marker of growth of tumor vessels. However, some opposite characteristics were found in the monolayers and tumors, including decreased proliferation, increased cell death, predominant G1 and G2/M cell cycle distribution and diminished sensitivity to cisplatin in cell cultures of lung epithelial cells while tumors had increased cell proliferation, decreased cell death, predominant S1 cell cycle distribution and exacerbated loss of cisplatin sensitivity.
The main concern of nanoscience is the toxicity associated with exposure to the engineered nanomaterials used for nanotechnology development, specifically, in occupational settings in which workers can be exposed continuously by inhalation and nanofibers have gained importance in applications related to waste water treatment, solar cells, bactericide devices, bone regeneration and conductors in lithium,59–62 which suggests that exposure in occupational settings might increase in the coming years. We have identified some complications in terms of occupational exposure limits for nanofibers. The first one is that recommendations given by different organizations do not differentiate between micro and nanosized particles, and the second one is that some other recommendations for instance, those given by the National Institute of Occupational Health and Safety establishes 0.3 mg m−3 as the limit for TiO2 exposure which includes micro and nanosized particles.63 The third complication is that some recommendations include all types of fibers without distinction between chemical compositions. For instance, the British Standard Institute establishes 0.01 fibers per cm3 as the limit for carbon nanotubes or metal oxide nanofibers, and the toxicity of nanoparticles is influenced not only by the size and shape but also is related to the chemical origin. Furthermore, respiratory protection in occupational settings is still inadequate because filtration systems for respirators only include protection for particles between 100–400 nm;64 however, the concern is related to particles sized below 100 nm. In addition, there is limited information about real exposure to nanofibers and it seems that it could occur under acute and chronic conditions at low and high levels at occupational places.
Regarding the xenograft in vivo model, the lack of an immune system allows the determination of the effect of exposure to TiO2 nanofibers in absence of inflammatory responses, which suggests that in the presence of an immune system, we speculate that macrophages and neutrophils, for instance, might release pro-inflammatory interleukins that are well-known to contribute to fibrosis development.13 In spite of lung epithelial cell cultures, there are lung adenocarcinoma derived cells that are useful for evaluating for instance EMT markers,65 sustained proliferation,66 cell migration and invasion.67
Taken together, we have observed that exposure to TiO2 nanofibers in monolayers induces some phenotypic characteristics in lung epithelial cells favoring tumor development in a xenograft model and these effects cannot be extrapolated to other types of NP fibers. The current study highlights the possibility that TiO2 nanofiber exposure in humans with previous diseases, especially cancer, could develop tumors with a more aggressive phenotype. Furthermore, the possibility that TiO2 nanofiber toxicity may be greater in lung epithelial cells than in phagocytic cells causing DNA damage and the fact that some types of lung cancer including non-small cell lung cancer begins in the epithelial cells highlights the necessity to investigate if cells exposed to TiO2 nanofibers promote a more aggressive cell phenotype.
5. Conclusion
The exposure to TiO2 nanofibers induced an increase in LDH release, a marker for cell cytotoxicity, after 7 days of exposure in lung epithelial cells. These cell cultures showed no alteration in cell proliferation, however, we found that genomic instability was detected as an increase in the frequency of trinucleated and tetranucleated cells, which is a clear footprint of DNA damage. Exposed monolayers to TiO2 nanofibers were positive for angiogenic, fibrotic and EMT markers, and those characteristics were maintained in the tumor xenograft model. In addition, tumors developed from TiO2 nanofiber exposed monolayers showed dedifferentiation and higher content of erythrocytes than tumors derived from control cells. TiO2 nanofibers induced loss of cisplatin sensitivity in monolayers and this effect was exacerbated in the tumors. These findings, altogether, demonstrate for the first time, that TiO2 nanofiber exposure in lung epithelial cells can enhance aggressive characteristics that have influence on tumor development.In addition, this study contributes to the understanding of effects associated with TiO2 nanofibers beyond cytotoxicity. We suggest that research on other cell types must be performed in order to determine the role of cell ontogeny on toxicity induced by TiO2 nanofiber exposure. For instance, in terms of inhalation, lung stem cells, lung fibroblasts, type I pneumocytes, and bronchial, bronchiolar, goblet and mesothelial cells exposed to TiO2 nanofibers are of crucial interest since they have several functions such as in tissue structure, secretion, movement, and immune response that might differentially respond against TiO2 nanofiber exposure.
We investigated the effects of TiO2 nanofiber exposure on cuboid epithelium (type II pneumocytes), however, research on other types such as columnar, stratified or transitional epithelium could clarify if the effects of these nanofibers have the same impact. This is relevant because 90% of the tumors originated from epithelial cells.
Finally, findings from this study must be restricted to this specific type of TiO2 nanofibers since no other nanofibers or nanoparticles were tested.
Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgements
This project was supported by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT IN218015) and the National Council of Science and Technology (CONACyT 268769). Medina-Reyes Estefany Ingrid is a doctoral student from Programa de Doctorado en Ciencias Biomédicas de la Universidad Nacional Autónoma de México (UNAM) and was supported by CONACYT (fellowship 576227). Déciga-Alcaraz Alejandro is a doctoral student from Programa de Doctorado en Ciencias Biomédicas de la Universidad Nacional Autónoma de México (UNAM) and was supported by CONACYT (fellowship 582547). We thank the Laboratorio Universitario de Caracterización Espectroscópica (LUCE) del Instituto de Ciencias Aplicadas y Tecnología (ICAT, UNAM) for the support in the nanofibers synthesis and Biol. Tomás Ernesto Villamar Duque for his help in the animal experimentation.References
- Nanowerk Nanomaterial Database™ https://www.nanowerk.com/nanomaterial-database.php Accessed Sep 18, 2018.
- USGS United States Geological Survey, Titanium and titanium dioxide. Mineral commodity summaries, 2013, pp. 172–173, https://minerals.usgs.gov/minerals/pubs/mcs/2000/mcs2000.pdf Accessed Sep 18, 2018 Search PubMed.
- F. Piccinno, F. Gottschalk, S. Seeger and B. Nowack, Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world, J. Nanopart. Res., 2012, 14, 1109 CrossRef.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Carbon black, titanium dioxide, and talc, IARC Monogr. Eval. Carcinog. Risks Hum., 2010, 93, 1–413 Search PubMed.
- S. Pavasupree, S. T. Dubas and R. Rangkupan, Surface modification of polypropylene non-woven fibers with TiO2 nanoparticles via layer-by-layer self assembly method: Preparation and photocatalytic activity, J. Environ. Sci., 2015, 37, 59–66 CrossRef CAS PubMed.
- S. Liu, L. Xie, J. Zheng, R. Jiang, F. Zhu, T. Luan and G. Ouyang, Mesoporous TiO2 nanoparticles for highly sensitive solid-phase microextraction of organochlorine pesticides, Anal. Chim. Acta, 2015, 878, 109–117 CrossRef CAS PubMed.
- Q. Guo, L. Liu, M. Zhang, H. Hou, Y. Song, H. Wang, B. Zhong and L. Wang, Hierarchically mesostructured porous TiO2 hollow nanofibers for high performance glucose biosensing, Biosens. Bioelectron., 2017, 92, 654–660 CrossRef CAS PubMed.
- K. Ulubayram, S. Calamak, R. Shahbazi and I. Eroglu, Nanofibers based antibacterial drug design, delivery and applications, Curr. Pharm. Des., 2015, 21(15), 1930–1943 CrossRef CAS.
- L. A. Jimenez, M. A. Gionet-Gonzales, S. Sedano, J. G. Carballo, Y. Mendez and W. Zhong, Extraction of microRNAs from biological matrices with titanium dioxide nanofibers, Anal. Bioanal. Chem., 2018, 410(3), 1053–1060 CrossRef CAS PubMed.
- C. Dumitriu, A. B. Stoian, I. Titorencu, V. Pruna, V. V. Jinga, R. M. Latonen, J. Bobacka and I. Demetrescu, Electrospun TiO2 nanofibers decorated Ti substrate for biomedical application, Mater. Sci. Eng., C, 2014, 45, 56–63 CrossRef CAS PubMed.
- E. Beltrán-Partida, B. Valdéz-Salas, A. Moreno-Ulloa, A. Escamilla, M. A. Curiel, R. Rosales-Ibáñez, F. Villarreal, D. M. Bastidas and J. M. Bastidas, Improved in vitro angiogenic behavior on anodized titanium dioxide nanotubes, J. Nanobiotechnol., 2017, 15(1), 10 CrossRef PubMed.
- L. A. Jimenez, M. A. Gionet-Gonzales, S. Sedano, J. G. Carballo, Y. Mendez and W. Zhong, Extraction of microRNAs from biological matrices with titanium dioxide nanofibers, Anal. Bioanal. Chem., 2018, 410(3), 1053–1060 CrossRef CAS PubMed.
- R. F. Hamilton, N. Wu, D. Porter, M. Buford, M. Wolfarth and A. Holian, Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity, Part. Fibre Toxicol., 2009, 6, 35 CrossRef.
- R. F. Hamilton, N. Wu, C. Xiang, M. Li, F. Yang, M. Wolfarth, D. W. Porter and A. Holian, Synthesis, characterization, and bioactivity of carboxylic acid-functionalized titanium dioxide nanobelts, Part. Fibre Toxicol., 2014, 11, 43 CrossRef.
- A. S. Reynolds, T. H. Pierre, R. McCall, J. Wu and W. E. Gato, Evaluating the cytotoxicity of tin dioxide nanofibers, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2018, 53(11), 986–991 CrossRef CAS PubMed.
- V. Rapisarda, C. Loreto, C. Ledda, G. Musumeci, M. Bracci, L. Santarelli, M. Renis, M. Ferrante and V. Cardile, Cytotoxicity, oxidative stress and genotoxicity induced by glass fibers on human alveolar epithelial cell line A549, Toxicol. In Vitro, 2015, 29(3), 551–557 CrossRef CAS PubMed.
- J. A. Tamminen, M. Myllärniemi, M. Hyytiäinen, J. Keski-Oja and K. Koli, Asbestos exposure induces alveolar epithelial cell plasticity through MAPK/Erk signaling, J. Cell. Biochem., 2012, 113(7), 2234–2247 CrossRef CAS PubMed.
- T. Chen, H. Nie, X. Gao, J. Yang, J. Pu, Z. Chen, X. Cui, Y. Wang, H. Wang and G. Jia, Epithelial-mesenchymal transition involved in pulmonary fibrosis induced by multi-walled carbon nanotubes via TGF-beta/Smad signaling pathway, Toxicol. Lett., 2014, 226(2), 150–162 CrossRef CAS PubMed.
- M. Allegri, M. G. Bianchi, M. Chiu, J. Varet, A. L. Costa, S. Ortelli, M. Blosi, O. Bussolati, C. A. Poland and E. Bergamaschi, Shape-Related Toxicity of Titanium Dioxide Nanofibres, PLoS One, 2016, 11(3), e0151365 CrossRef PubMed.
- D. W. Porter, N. Wu, A. F. Hubbs, R. R. Mercer, K. Funk, F. Meng, J. Li, M. G. Wolfarth, L. Battelli, S. Friend, M. Andrew, R. Hamilton Jr, K. Sriram, F. Yang, V. Castranova and A. Holian, Differential mouse pulmonary dose and time course responses to titanium dioxide nanospheres and nanobelts, Toxicol. Sci., 2013, 131(1), 179–193 CrossRef CAS PubMed.
- E. I. Medina-Reyes, A. Déciga-Alcaraz, V. Freyre-Fonseca, N. L. Delgado-Buenrostro, J. O. Flores-Flores, G. F. Gutiérrez-López, Y. Sánchez-Pérez, C. M. García-Cuéllar, J. Pedraza-Chaverri and Y. I. Chirino, Titanium dioxide nanoparticles induce an adaptive inflammatory response and invasion and proliferation of lung epithelial cells in chorioallantoic membrane, Environ. Res., 2015, 136, 424–434 CrossRef CAS PubMed.
- Y. Nie, Y. Li and S. Hu, A novel small inhibitor, LLL12, targets STAT3 in non-small cell lung cancer in vitro and in vivo, Oncol. Lett., 2018, 16(4), 5349–5354 Search PubMed.
- F. X. Huang, H. J. Chen, F. X. Zheng, Z. Y. Gao, P. F. Sun, Q. Peng, Y. Liu, X. Deng, Y. H. Huang, C. Zhao and L. J. Miao, LncRNA BLACAT1 is involved in chemoresistance of non-small cell lung cancer cells by regulating autophagy, Int. J. Oncol., 2018, 54(1), 339–347 Search PubMed.
- N. A. Hotaling, K. Bharti, H. Kriel and C. G. Simon Jr, Diameter J: A validated open source nanofiber diameter measurement tool, Biomaterials, 2015, 61, 327–338 CrossRef CAS PubMed.
- Y. Shintani, M. Maeda, N. Chaika, K. R. Johnson and M. J. Wheelock, Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signaling, Am. J. Respir. Cell Mol. Biol., 2008, 38(1), 95–104 CrossRef CAS.
- Y. Yu, Y. Lin, G. Yang and L. Tian, The interplay between TGF-β/SMAD and BMP/SMAD signaling pathways in the epithelial mesenchymal transition of A549 cells induced by silica, Toxicol. Mech. Methods, 2018, 28(4), 286–292 CrossRef CAS PubMed.
- M. Fenech, The in vitro micronucleus technique, Mutat. Res., 2000, 455(1–2), 81–95 CrossRef CAS.
- S. Reddy, D. Piccione, H. Takita and R. B. Bankert, Human lung tumor growth established in the lung and subcutaneous tissue of mice with severe combined immunodeficiency, Cancer Res., 1987, 47(9), 2456–2460 CAS.
- M. Jakubowska, M. Sniegocka, E. Podgórska, D. Michalczyk-Wetula, K. Urbanska, A. Susz, L. Fiedor, J. Pyka and P. M. Płonka, Pulmonary metastases of the A549-derived lung adenocarcinoma tumors growing in nude mice, A multiple case study, Acta Biochim. Pol., 2013, 60(3), 323–330 CAS.
- C. H. Ma, R. Jiang, J. D. Li, B. Wang, L. W. Sun and Y. Lv, Experimental study of Endostar injection concomitant with cryoablation on lung adenocarcinoma A549 xenografts, Asian Pac. J. Cancer Prev., 2013, 14(11), 6697–6701 CrossRef.
- W. Nuansing, S. Ninmuang, W. Jarernboon, S. Maensiri and S. Seraphin, Structural characterization and morphology of electrospun TiO2 nanofibers, Mater. Sci. Eng., B, 2006, 131(1–3), 147–155 CrossRef CAS.
- M. Wang and N. O. Petersen, Lipid-coated gold nanoparticles promote lamellar body formation in A549 cells, Biochim. Biophys. Acta, 2013, 1831(6), 1089–1097 CrossRef CAS PubMed.
- V. Kononenko, A. Erman, T. Petan, I. Križaj, S. Kralj, D. Makovec and D. Drobne, Harmful at non-cytotoxic concentrations: SiO2-SPIONs affect surfactant metabolism and lamellar body biogenesis in A549 human alveolar epithelial cells, Nanotoxicology, 2017, 11(3), 419–429 CrossRef CAS PubMed.
- J. R. Glasser and R. K. Mallampalli, Surfactant and its role in the pathobiology of pulmonary infection, Microbes Infect., 2012, 14(1), 17–25 CrossRef CAS PubMed.
- R. Ghidoni, A. Caretti and P. Signorelli, Role of Sphingolipids in the Pathobiology of Lung Inflammation, Mediators Inflammation, 2015, 2015, 487508 Search PubMed.
- J. Ma, B. Bishoff, R. R. Mercer, M. Barger, D. Schwegler-Berry and V. Castranova, Role of epithelial-mesenchymal transition (EMT) and fibroblast function in cerium oxide nanoparticles-induced lung fibrosis, Toxicol. Appl. Pharmacol., 2017, 323, 16–25 CrossRef CAS PubMed.
- M. Watanabe, M. Okada, Y. Kudo, Y. Tonori, M. Niitsuya, T. Sato, Y. Aizawa and M. Kotani, Differences in the effects of fibrous and particulate titanium dioxide on alveolar macrophages of Fischer 344 rats, J. Toxicol. Environ. Health, Part A, 2002, 65(15), 1047–1060 CrossRef CAS PubMed.
- X. Jin, Y. Aimaiti, Z. Chen, W. Wang and D. Li, Hepatic stellate cells promote angiogenesis via the TGF-β1-Jagged1/VEGFA axis, Exp. Cell Res., 2018, 373(1–2), 34–43 CrossRef CAS PubMed.
- K. Miyazono, Y. Katsuno, D. Koinuma, S. Ehata and M. Morikawa, Intracellular and extracellular TGF-β signaling in cancer: some recent topics, Front. Med., 2018, 12(4), 387–411 CrossRef PubMed.
- Y. Huang, G. Li, K. Wang, Z. Mu, Q. Xie, H. Qu, H. Lv and B. Hu, Collagen Type VI Alpha 3 Chain Promotes Epithelial-Mesenchymal Transition in Bladder Cancer Cells via Transforming Growth Factor β (TGF-β)/Smad Pathway, Med. Sci. Monit., 2018, 24, 5346–5354 CrossRef PubMed.
- A. A. Ezhilarasi, J. J. Vijaya, K. Kaviyarasu, M. Maaza, A. Ayeshamariam and L. J. Kennedy, Green synthesis of NiO nanoparticles using Moringa oleifera extract and their biomedical applications: Cytotoxicity effect of nanoparticles against HT-29 cancer cells, J. Photochem. Photobiol., B, 2016, 164, 352–360 CrossRef CAS PubMed.
- A. A. Mobeen, S. S. K. Jasmine, R. Sundaram, M. C. Maria, K. Kaviyarasu, D. Letsholathebe, S. B. Mohamed, J. Kennedy and M. Maaza, Antibacterial, magnetic, optical and humidity sensor studies of β-CoMoO4 - Co3O4 nanocomposites and its synthesis and characterization, J. Photochem. Photobiol., B, 2018, 183, 233–241 CrossRef PubMed.
- M. C. Maria, K. Kaviyarasu, A. Raja, M. V. Arularasu, G. T. Mola, A. B. Isaev, N. A. Al-Dhabi, M. V. Arasu, B. Jeyaraj, J. Kennedy and M. Maaza, Photocatalytic decomposition effect of erbium doped cerium oxide nanostructures driven by visible light irradiation: Investigation of cytotoxicity, antibacterial growth inhibition using catalyst, J. Photochem. Photobiol., B, 2018, 185, 275–282 CrossRef PubMed.
- W. E. Gato, D. A. Hunter, I. C. Byrd, C. A. Mays, W. Yau and J. Wu, Assessment of the short-term toxicity of TiO2 nanofiber in Sprague Dawley rats, Environ. Toxicol., 2017, 32(6), 1775–1783 CrossRef CAS PubMed.
- K. M. Ramkumar, C. Manjula, G. Gnanakumar, M. A. Kanjwal, T. V. Sekar, R. Paulmurugan and P. Rajaguru, Oxidative stress-mediated cytotoxicity and apoptosis induction by TiO2 nanofibers in HeLa cells, Eur. J. Pharm. Biopharm., 2012, 81(2), 324–333 CrossRef CAS PubMed.
- L. K. Bartel, D. A. Hunter, K. B. Anderson, W. Yau, J. Wu and W. E. Gato, Short-term evaluation of hepatic toxicity of titanium dioxide nanofiber (TDNF), Drug Chem. Toxicol., 2018, 1–8 CrossRef PubMed.
- H. Proquin, C. Rodríguez-Ibarra, C. G. Moonen, I. M. Urrutia Ortega, J. J. Briedé, T. M. de Kok, H. van Loveren and Y. I. Chirino, Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: contribution of micro and nano-sized fractions, Mutagenesis, 2017, 32(1), 139–149 CrossRef CAS PubMed.
- H. Proquin, M. J. Jetten, M. C. M. Jonkhout, L. G. Garduño-Balderas, J. J. Briedé, T. M. de Kok, Y. I. Chirino and H. van Loveren, Gene expression profiling in colon of mice exposed to food additive titanium dioxide (E171), Food Chem. Toxicol., 2018, 111, 153–165 CrossRef CAS PubMed.
- D. M. Jensen, D. V. Christophersen, M. Sheykhzade, G. F. Skovsted, J. Lykkesfeldt, R. Münter, M. Roursgaard, S. Loft and P. Møller, Vasomotor function in rat arteries after ex vivo and intragastric exposure to food-grade titanium dioxide and vegetable carbon particles, Part. Fibre Toxicol., 2018, 15(1), 12 CrossRef PubMed.
- L. Shi, N. Dong, X. Fang and X. Wang, Regulatory mechanisms of TGF-β1-induced fibrogenesis of human alveolar epithelial cells, J. Cell. Mol. Med., 2016, 20(11), 2183–2193 CrossRef CAS PubMed.
- B. Rybinski, J. Franco-Barraza and E. Cukierman, The wound healing, chronic fibrosis, and cancer progression triad, Physiol. Genomics, 2014, 46(7), 223–244 CrossRef CAS.
- D. Avery, P. Govindaraju, M. Jacob, L. Todd, J. Monslow and E. Puré, Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts, Matrix Biol., 2018, 67, 90–106 CrossRef CAS PubMed.
- T. Xia, R. F. Hamilton, J. C. Bonner, E. D. Crandall, A. Elder, F. Fazlollahi, T. A. Girtsman, K. Kim, S. Mitra, S. A. Ntim, G. Orr, M. Tagmount, A. J. Taylor, D. Telesca, A. Tolic, C. D. Vulpe, A. J. Walker, X. Wang, F. A. Witzmann, N. Wu, Y. Xie, J. I. Zink, A. Nel and A. Holian, Interlaboratory evaluation of in vitro cytotoxicity and inflammatory responses to engineered nanomaterials: the NIEHS Nano GO Consortium, Environ. Health Perspect., 2013, 121(6), 683–690 CrossRef PubMed.
- K. Donaldson, F. Murphy, A. Schinwald, R. Duffin and C. A. Poland, Identifying the pulmonary hazard of high aspect ratio nanoparticles to enable their safety-by-design, Nanomedicine, 2011, 6(1), 143–156 CrossRef CAS PubMed.
- J. K. Thompson, M. B. MacPherson, S. L. Beuschel and A. Shukla, Asbestos-Induced Mesothelial to Fibroblastic Transition Is Modulated by the Inflammasome, Am. J. Pathol., 2017, 187(3), 665–678 CrossRef CAS PubMed.
- H. Tan, S. Zhang, H. Liu, W. Peng, R. Li, Y. Gu, X. Wang, J. Mao and X. Shen, Imaging findings in phyllodes tumors of the breast, Eur. J. Radiol., 2012, 81(1), 62–69 CrossRef PubMed.
- G. Bredholt, M. Mannelqvist, I. M. Stefansson, E. Birkeland, T. H. Bø, A. M. Øyan, J. Trovik, K. H. Kalland, I. Jonassen, H. B. Salvesen, E. Wik and L. A. Akslen, Tumor necrosis is an important hallmark of aggressive endometrial cancer and associates with hypoxia, angiogenesis and inflammation responses, Oncotarget, 2015, 6(37), 39676–39691 CrossRef PubMed.
- S. Y. Lee, M. K. Ju, H. M. Jeon, E. K. Jeong, Y. J. Lee, C. H. Kim, H. G. Park, S. I. Han and H. S. Kang, Regulation of Tumor Progression by Programmed Necrosis, Oxid. Med. Cell. Longevity, 2018, 2018, 3537471 Search PubMed.
- K. S. Brammer, C. J. Frandsen and S. Jin, TiO2 nanotubes for bone regeneration, Trends Biotechnol., 2012, 30(6), 315–322 CrossRef CAS PubMed.
- J. S. Cho, Y. J. Hong and Y. C. Kang, Electrochemical properties of fiber-in-tube- and filled-structured TiO2 nanofiber anode materials for lithium-ion batteries, Chemistry, 2015, 21(31), 11082–11087 CrossRef CAS PubMed.
- A. Mohamed, R. El-Sayed, T. A. Osman, M. S. Toprak, M. Muhammed and A. Uheida, Composite nanofibers for highly efficient photocatalytic degradation of organic dyes from contaminated water, Environ. Res., 2016, 145, 18–25 CrossRef CAS PubMed.
- M. Batmunkh, T. J. Macdonald, C. J. Shearer, M. Bat-Erdene, Y. Wang, M. J. Biggs, I. P. Parkin, T. Nann and J. G. Shapter, Carbon Nanotubes in TiO2 Nanofiber Photoelectrodes for High-Performance Perovskite Solar Cells, Adv. Sci., 2017, 4(4), 1600504 CrossRef PubMed.
- National Institute for Occupational Safety and Health (NIOSH), Current Intelligence Bulletin 63: Occupational Exposure to Titanium Dioxide Department of Health and Human Services (NIOSH) Publication Number 2011-160. https://www.cdc.gov/niosh/docs/2011-160/pdfs/2011-160.pdf, accessed Sep 18, 2018.
- E. Vo, Z. Zhuang, M. Horvatin, Y. Liu, X. He and S. Rengasamy, Respirator Performance against Nanoparticles under Simulated Workplace Activities, Ann. Occup. Hyg., 2015, 59(8), 1012–1021 CrossRef PubMed.
- A. Zahedi, R. Phandthong, A. Chaili, G. Remark and P. Talbot, Epithelial-to-mesenchymal transition of A549 lung cancer cells exposed to electronic cigarettes, Lung Cancer, 2018, 122, 224–233 CrossRef PubMed.
- V. Mucchietto, F. Fasoli, S. Pucci, M. Moretti, R. Benfante, A. Maroli, S. Di Lascio, C. Bolchi, M. Pallavicini, C. Dowell, M. McIntosh, F. Clementi and C. Gotti, α9- and α7-containing receptors mediate the pro-proliferative effects of nicotine in the A549 adenocarcinoma cell line, Br. J. Pharmacol., 2018, 175(11), 1957–1972 CrossRef CAS PubMed.
- Y. Wang, W. Zhai, H. Wang, X. Xia and C. Zhang, Benzo(a)pyrene promotes A549 cell migration and invasion through up-regulating Twist, Arch. Toxicol., 2015, 89(3), 451–458 CrossRef CAS PubMed.
Footnote |
| † Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México. |
| This journal is © The Royal Society of Chemistry 2019 |