Photolysis of graphene oxide in the presence of nitrate: implications for graphene oxide integrity in water and wastewater treatment†
Lin
Duan
a,
Tong
Zhang
a,
Weihua
Song
b,
Chuanjia
Jiang
a,
Yan
Hou
a,
Weilu
Zhao
a,
Wei
Chen
 *a and
Pedro J. J.
Alvarez
c
*a and
Pedro J. J.
Alvarez
c
aCollege of Environmental Science and Engineering, Ministry of Education Key Laboratory of Pollution Processes and Environmental Criteria, Tianjin Key Laboratory of Environmental Remediation and Pollution Control, Nankai University, 38 Tongyan Road, Tianjin 300350, China. E-mail: chenwei@nankai.edu.cn; Fax: +86 22 85358169; Tel: +86 22 85358169
bDepartment of Environmental Science & Engineering, Fudan University, 220 Handan Road, Shanghai 200433, China
cDepartment of Civil and Environmental Engineering, Rice University, 6100 Main Street, Houston, Texas 77005, USA
First published on 2nd November 2018
Abstract
Despite the widespread use of graphene oxide (GO) in diverse applications and increasing interest in its inclusion in some water treatment devices, mechanistic understanding of photochemical GO transformations is limited. This is an important knowledge gap relevant to GO performance and durability. We examined the reaction pathways and products of a GO suspension under UV irradiation in the presence of nitrate, a common anion in water and wastewater treatment processes. As the nitrate concentration increased, the dominant pathway of GO transformation changed from direct photolysis to indirect photolysis enhanced by the production of hydroxyl radicals (˙OH) during UV irradiation of nitrate. At environmentally relevant concentrations (e.g., 1 mM), nitrate induced significant fragmentation of the GO nanostructure. The significant effects of ˙OH on GO morphology and surface properties were verified by negative-control tests, including deoxygenation of the suspension, reactive oxygen species (ROS) inhibition and radical trapping, and by γ-radiolysis, known to generate a single ROS: ˙OH. Supplemental photolysis experiments conducted using graphite demonstrated that the main reaction pathways of the indirect photolysis of GO not only include the oxidation reactions between ˙OH and the oxidized domains of GO, but also the electrophilic addition reaction between ˙OH and the aromatic domains. These findings have significant implications for GO integrity and durability in systems involving incidental or purposeful exposure to UV irradiation.
Environmental significanceGraphene oxide (GO) materials are a promising class of carbonaceous nanomaterials that hold great promise for enhancing water and wastewater treatment (e.g., membranes and adsorbents). When used in treatment processes, GO materials may be exposed to various chemical and biological agents, resulting in alterations of their morphologies and surface properties. This may affect not only the performances of GO-based materials, but also their integrity and durability. This study shows that the presence of nitrate, one of the most common inorganic anions in water and wastewater, at environmentally relevant concentrations significantly enhances the photolysis of GO materials, resulting in disintegration of GO nanosheets, as well as substantial alterations of GO surface functional groups. These results underscore the need to understand the photochemical transformation mechanisms of carbonaceous nanomaterials and the associated implications for their performance and durability. |
1 Introduction
Graphene oxide (GO) is one of the promising carbonaceous nanomaterials of significant interest for a number of applications, such as polymer composite fabrication, electronic devices, energy storage, and biomedicine.1–3 Owing to its high surface area and abundant surface O-functional groups, GO has also been considered to improve water and wastewater treatment devices such as adsorbents, catalysts, and filtration membranes.4–9 Moreover, a variety of metal/metal oxide nanoparticles and polymers can be anchored to GO to achieve higher efficiency for contaminant removal.10 During applications in water or wastewater treatment, GO will likely be exposed to various chemical and biological agents, which may result in the transformation of GO11–13 and compromise the performance, integrity, and durability of these materials. Transformation of GO may also result in the formation of toxic contaminants, such as oxygenated polycyclic aromatic hydrocarbon species.14,15Ultraviolet (UV) irradiation, a commonly adopted approach for disinfection and contaminant degradation,16 has been shown to induce photochemical transformation of GO.15,17–25 Photolysis of GO under UV irradiation may occur through both direct and indirect pathways.15,18–25 GO can undergo direct photolysis by acting similarly to photo-reactive semiconductors to generate electron–hole pairs, leading to the oxidation of O-functional groups to a higher oxidation state (quinones and carboxylic acids) by the holes or the reduction of O-functional groups by trapped-electrons, finally resulting in the loss of O-containing functional groups and the appearance of cavities and defects on GO nanosheets.19–22,25 A limited number of studies have also been conducted to understand indirect photolysis of GO. It was reported that GO can undergo indirect photolysis in the presence of H2O2 or Fenton reagents (Fe2+/Fe3+/H2O2).15,23,24 However, while external reactive oxygen species (ROS) was assumed to play an important role in indirect photolysis of GO, little is known about the photolysis products of GO by external ROS source reagents at environmentally relevant concentrations, and the relative contribution of direct vs. indirect photolysis in such systems. Moreover, the specific reactivity of different GO domains toward ROS is unclear. For instance, it has been proposed that indirect photolysis of GO by ROS is mainly due to the oxidation reactions between ROS and the oxidized domains, and the reaction rates depend strongly on the oxidation degree of GO.15,23–26 Nonetheless, other researchers proposed that the pristine sp2-carbon structures are the primary active sites to scavenge radicals, based on the observed antioxidant effects of GO.27
Natural water and wastewater often contains complex constituents (e.g., natural organic matter and inorganic anions) that can induce the generation of ROS.28–31 One of the important precursors for these ROS is nitrate, a common inorganic anion in water as a consequence of agricultural application of nitrogenous fertilizers and manures as well as the discharge of industrial nitrogenous wastes.32,33 The maximum contaminant level (MCL) for nitrate is 10 mg L−1 nitrate-nitrogen in drinking water supply, but nitrate concentration can reach much higher levels, with concentrations up to hundreds of mg L−1 in groundwater of agriculture areas.29,34 Many ROS, such as nitrogen oxide radicals (˙NO and ˙NO2), superoxide radical anion (O2˙−) and hydroxyl radical (˙OH), can be generated when nitrate is exposed to UV.29,32,35,36 Thus, we postulate that when GO is exposed to UV irradiation in the presence of nitrate, the radicals generated may result in indirect photolysis of GO. To date, the potential effects of nitrate-induced indirect GO photolysis, at environmentally relevant nitrate concentrations, on GO physicochemical properties have not been investigated.
This study seeks to advance mechanistic understanding of GO photolysis and reaction pathways in the presence of nitrate. The effects of nitrate were examined by comparing changes in morphology, structure and surface O-functional groups of GO induced by UV treatment in the presence and absence of nitrate, using combined spectroscopic techniques. Different nitrate concentrations were tested in photolysis experiments to identify the relative contribution of indirect photolysis of GO in the presence of nitrate. Anaerobic experiments, ROS inhibition experiments and radical trapping were conducted as negative controls and γ-radiolysis experiments as a positive control to discern the predominant reactive species responsible for the observed nitrate effects. Moreover, functionality-free, pure graphite was used as a model material to identify the reactivity of the aromatic domains of GO during the indirect photolysis of GO.
2 Experimental
2.1 Materials
GO (>99%) was purchased from Nano Materials Tech Co. (Tianjin, China). According to the supplier, the product was synthesized from graphite using a modified Hummers method. Pure graphite (99.99%) was purchased from Sigma Aldrich (St. Louis, MO, USA). Sodium nitrate (NaNO3), sodium sulfate (Na2SO4) and sodium chloride (NaCl) of chemical grade were obtained from Guangfu Tech Co. (Tianjin, China). Isopropylamine and parachlorobenzoic acid (pCBA) was purchased from Mackin biochemical Co. (Shanghai, China). Humic acid (HA) was purchased from Guangfu Tech Co. (Tianjin, China).2.2 Photolysis experiments
An aqueous stock suspension of GO was prepared using the following procedures according to our previous study.37 First, approximately 60 mg of GO powder was added to 300 mL of deionized (DI) water and mixed using a magnetic stir bar for 0.5 h, and then the mixture was sonicated (600 W, 40 MHz, SB25-12DTDN, Sicentzbio Tech Co. Ningbo, China) for 4 h in a water bath at 30 °C. Finally, the well-mixed GO suspension was kept in dark at 4 °C until use. The working GO suspension was prepared for each irradiation experiment by diluting the GO stock suspension with DI water to a concentration of 10 mg L−1.Photolysis experiments were carried out in 60 mL customized quartz tubes using a XPA-7 photoreactor (Xujiang, Nanjing, China) equipped with a 500 W medium pressure mercury (Hg) lamp in the center of the photoreactor. To initiate a photolysis experiment, 50 mL of 10 mg L−1 GO suspension was added in the quartz tube with or without sodium salts (NaNO3, Na2SO4 and NaCl) and then sealed with a ground glass stopper and completely mixed using a magnetic stir bar in the dark for 1 h. The reactor was submerged in a thermostatic water bath (25 °C), and exposed to the 500 W medium pressure Hg lamp. The spectrum of UV light emitted from the Hg lamp was measured with a radiometer (RPS900-R, International Light, USA) and presented in the ESI† (Fig. S1). At determined time periods during irradiation, GO suspension in the quartz tube was removed from the reactor and sacrificed for analysis. To test the effects of nitrate, stock solution of different sodium salts (NaNO3, Na2SO4 and NaCl) was added to the working GO suspension to reach a final anion concentration of 1 mM. NaNO3 was also added at concentrations of 0.05, 0.1 and 5 mM to identify the effects of nitrate concentration on GO photolysis. To examine the effects of variable water solution chemistry on GO transformation in the presence of nitrate, additional photolysis experiments were carried out by adding different concentrations (1, 5, 10, 50 or 100 mg L−1) of humic acid (as representative natural organic matter), and by using tap water or pond water.
For experiments under anaerobic conditions, GO suspension (50 mL) was purged with high-purity N2 or Ar gas for at least 1 h and sealed to achieve a dissolved O2-deficient environment prior to UV irradiation.38,39 To identify the ROS during UV irradiation in the presence of nitrate, 1% (v/v) isopropylamine and 0.5 ppm pCBA were used as a scavenger and a probe for ˙OH, respectively. All experiments conducted under UV-irradiation were performed in duplicate. Additionally, 10 mg L−1 GO aqueous suspension saturated with nitrous oxide (N2O) was subjected to different doses of gamma (γ) radiation (400, 800, 1600 and 3000 Gy) to further identify the role of ˙OH in GO photolysis,24,25 using 137Cs γ-rays (γ-Cell 40, Atomic Energy of Canada, Ontario, Canada) at a dose rate of 1.0 Gy min−1.
2.3 Material characterization
The morphologies and structures of GO samples were characterized by transmission electron microscopy (TEM) (FEI Tecnai G2100 F-20, Hatfield, PA) and atomic force microscopy (AFM) (Veeco Multimode Nanoscope VIII, Santa Barbara, CA). The structural changes of GO after UV irradiation were monitored by UV-vis absorption (UV-2401 UV-vis spectrophotometer, Shimadzu, Japan), X-ray diffraction (XRD) (Rigaku D/max-2500, Japan) and Raman spectroscopy (Renishaw inVia Raman microscope, RM2000, UK). Surface chemistry properties of GO were characterized by X-ray photoelectron spectroscopy (XPS) (PHI 5000 VersaProbe, Japan). The photolysis products of GO were analyzed using electrospray ionization-mass spectrometry (ESI-MS, Xevo TQ-S, Waters, USA). Total organic carbon (TOC) concentrations were determined with a high sensitivity TOC analyzer (Shimadzu Scientific Instruments, Columbia, MD). Surface O-functional groups of the photo-transformed graphite were characterized by Fourier transform infrared (FTIR) transmission spectroscopy (110 Bruker TENSOR 27 apparatus, Germany).3 Results and discussion
3.1 Photo-transformation of GO affected by nitrate
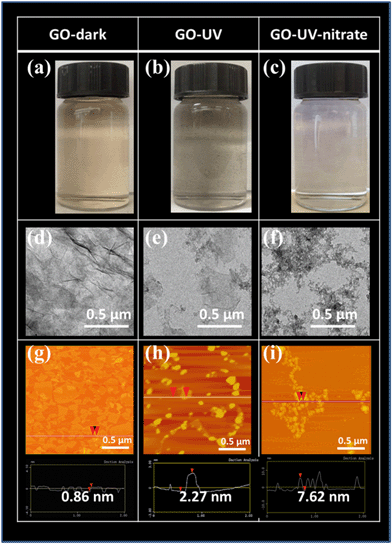
TEM images showed significant damages of GO nanosheets for GO-UV-nitrate, wherein the smooth basal plane of GO nanosheets was disintegrated into small fragments (Fig. 1f). In contrast, GO-UV, GO-UV-sulfide and GO-UV-chloride showed larger pieces with irregularly shaped GO nanoflakes (Fig. 1e and ESI† Fig. S2e and f). The AFM images corroborated the significant effects of nitrate on GO photolysis (Fig. 1g–i, and ESI† Fig. S2g–i and S5). For GO-dark, the lateral sizes of GO nanosheets showed a broad distribution and the thickness of the nanosheets was mostly below 1 nm (Fig. 1g and ESI† Fig. S5); for GO-UV, GO nanoflakes with dominant square root of areas of 50–100 nm and thickness of 5–10 nm (Fig. 1h and ESI† Fig. S5) were observed, likely attributable to the aggregation of the photoreaction products. In comparison, GO-UV-nitrate consisted of numerous small-sized fragments with dominant square root of areas below 50 nm and thickness of 5–15 nm (Fig. 1i and ESI† Fig. S5), indicating the significant disintegration of GO flakes and increased tendency of aggregation.
The structural changes of GO from photolysis were further characterized by Raman and XRD analyses (ESI† Fig. S8). Even though UV irradiation both in the absence and presence of nitrate resulted in the reduction of GO as evidenced by the XRD patterns of GO-UV and GO-UV-nitrate, the Raman spectra showed that GO-UV-nitrate had a larger ID/IG (the ratio of the intensities of the D and G bands) value than did GO-UV, indicating a more significant decrease in the average size of the π-conjugated structures of GO nanosheets and an increase in the abundance of GO edges or defects when GO was UV-irradiated in the presence of nitrate.1,15,20
A series of low molecular-weight species as the photolysis products were detected in the mass spectrometry (MS) spectra of both GO-UV and GO-UV-nitrate (ESI† Fig. S9), indicating fragmentation of GO nanosheets; this is consistent with the findings of Hou et al.14 However, for GO-UV-nitrate, the photolysis products in the higher mass-to-charge ratio (m/z) range (i.e., 700–1000) almost disappeared, whereas these products remained for GO-UV. This further corroborated the more significant fragmentation of GO nanostructure for GO-UV-nitrate than that for GO-UV. This observation may have important implications as small toxic organic molecules (e.g., oxygenated polycyclic aromatic hydrocarbon species) might be formed from this process.14,15 Consistently, a significant decrease of TOC concentration with irradiation time was observed for the experiment involving nitrate (ESI† Fig. S10), indicating that extensive degradation of GO occurred.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C increased and intensity of C–O decreased for both GO-UV and GO-UV-nitrate (Table 1 and ESI† Fig. S11), consistent with previous studies on UV induced GO transformation.19,20 Notably, GO-UV-nitrate contained markedly greater amount of C–O (20.59%) than GO-UV (14.18%), but significantly smaller amount of O–C
C increased and intensity of C–O decreased for both GO-UV and GO-UV-nitrate (Table 1 and ESI† Fig. S11), consistent with previous studies on UV induced GO transformation.19,20 Notably, GO-UV-nitrate contained markedly greater amount of C–O (20.59%) than GO-UV (14.18%), but significantly smaller amount of O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (4.96%) than GO-UV (10.68%), indicating that the presence of nitrate resulted in surface oxide formation of GO.
O (4.96%) than GO-UV (10.68%), indicating that the presence of nitrate resulted in surface oxide formation of GO.
| Sample ID | C species (wt%) | C/O ratio | |||
|---|---|---|---|---|---|
C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
C–O | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
||
| a “GO-dark” represents the GO sample without UV irradiation (kept in dark condition). b “GO-UV” represents the GO product after 9 h of UV irradiation in DI water. c “GO-UV-nitrate” represents the GO product after 9 h of UV irradiation in the presence of nitrate (1 mM). | |||||
| GO-darka | 46.67 | 40.59 | 10.31 | 2.42 | 2.40 |
| GO-UVb | 72.89 | 14.18 | 2.23 | 10.68 | 4.28 |
| GO-UV-nitratec | 70.85 | 20.59 | 3.60 | 4.96 | 3.93 |
3.2 Identification of ROS responsible for nitrate-induced indirect photolysis of GO
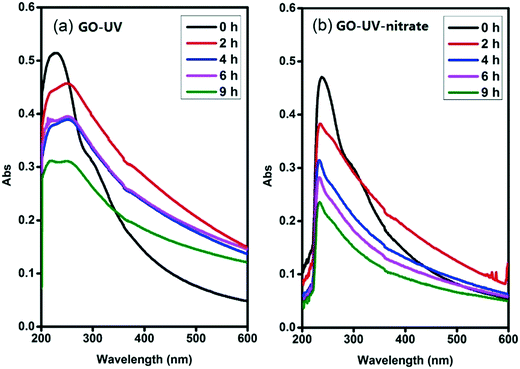
Photolysis of nitrate is known to produce different ROS, including ˙NO or ˙NO2, O2˙− and ˙OH,29,32,35,36 which may contribute to the indirect photolysis of GO. Nitrogen oxide radicals (˙NO and ˙NO2) are weaker oxidants compared to ˙OH, and can be rapidly recombined to form nitrate.35,36 Accordingly, these two ROS likely were not important species responsible for the significant indirect photolysis of GO. Since deoxygenation of the reaction matrix did not inhibit the significant disintegration of GO sheets (Fig. 4a and ESI† Fig. S12), O2˙− likely was not an important ROS responsible to the observed effects of nitrate on GO transformation. Interestingly, the addition of 1% isopropylamine, a scavenger of ˙OH,41 greatly inhibited the disintegration of GO nanosheets (Fig. 4b), as the color of the suspension turned blackish instead of becoming clear, similar to the appearance of the suspension of GO-UV (Fig. 1b). This result indicated that the nitrate-induced indirect photolysis of GO was mainly caused by the photo-generated ˙OH.To further verify that ˙OH was the predominant ROS responsible for the nitrate-induced indirect photolysis of GO, we conducted supplemental experiments using N2O-saturated water under steady-state γ-radiation, a condition known to generate only ˙OH.26,42 The color of the GO suspension became gradually transparent when receiving increasing doses of γ-radiation (400, 800, 1600 and 3000 Gy, Fig. 4c) (i.e., the concentration of ˙OH increased with the increasing dose of γ-radiation). Consistently, the characteristic peak of GO centered at 230 nm of the UV-vis spectrum gradually disappeared during γ-radiolysis (ESI† Fig. S13), indicating molecular alteration due to the reaction of GO with ˙OH.43 These trends are consistent with that observed for GO-UV-nitrate (Fig. 1c), confirming that nitrate-induced indirect photolysis of GO occurred primarily through a ˙OH-induced pathway.
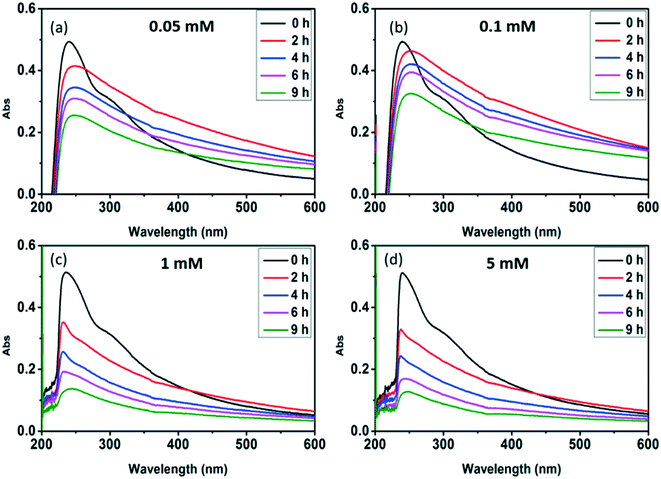
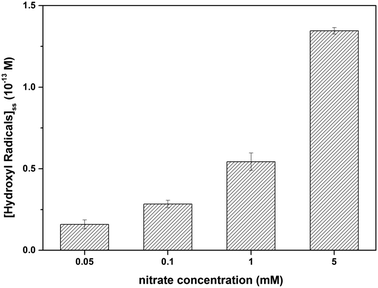
Radical trapping experiment using pCBA as the ˙OH probe showed that the steady-state concentrations of ˙OH ([˙OH]ss) in the GO suspension during UV-irradiation with nitrate increased with increasing nitrate concentration (from 1.6 × 10−14 M for 0.05 mM nitrate to 1.35 × 10−13 M for 5 mM nitrate) (Fig. 5), indicating that the generation of ˙OH was dependent on nitrate concentration and was consistent with the concentration-dependent effects of nitrate on GO transformation (Fig. 3). Note that the amount of ˙OH generated in the system containing only GO (i.e., in the absence of nitrate) can be neglected when compared with the systems containing both GO and nitrate (Fig. 5), which was likely related to the inhibition of ˙OH through UV absorption and scavenging of ˙OH by GO. This is consistent with previous research by Zhao and Jafvert41 showing that upon solar light irradiation of single layered GO dispersed in water, only a negligible amount of ˙OH was detected, mainly because the scavenging of ˙OH by GO was likely rapid and significant.
3.3 Proposed reaction pathways for nitrate-induced indirect photolysis of GO
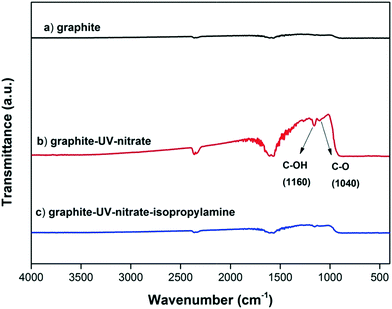
GO contains both oxidized domains and aromatic domains.44 It has been proposed that the indirect photolysis of GO occurs mainly through the oxidation reactions between ˙OH and the O-functional groups of GO, and the reaction rate depends strongly on the oxidization extent of GO.15,23,36 However, it has also been reported that the aromatic domains of GO were the primary site to scavenge ROS,27 suggesting that the oxidation reaction likely occurred at the aromatic domains of GO.27 Consistently, we hypothesize that the aromatic domains are also susceptible to the oxidation by ˙OH.To determine whether the aromatic domains of GO can directly react with ˙OH during the indirect photolysis of GO, an ROS inhibition experiment using isopropylamine as ˙OH scavenger was conducted using the functionality-free, pure graphite (99.99% C) as a model material. As expected, FTIR analysis showed that in the presence of nitrate, the absorption bands around 1160 cm−1 and 1040 cm−1 (which are ascribed to C–OH and C–O) appeared on graphite upon UV irradiation,45 indicating that the O-functionality-free aromatic structures of graphite were reactive toward ˙OH; however, when isopropylamine was added, no observable amounts of O-functional groups were formed on graphite surfaces (Fig. 6). These results support the hypothesis that ˙OH oxidized the aromatic domains of GO.
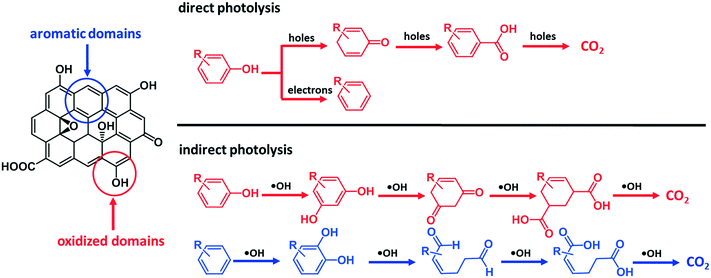
On the basis of these observations and the literature,46 we postulate that ˙OH reacted with the aromatic rings of GO surface via electrophilic addition reactions, forming hydroxylated GO, which underwent further oxidation. The direct attack of ˙OH on the π-conjugated structures of GO can cause ring cleavage of the aromatic moieties, in a similar way to the ring cleavage process that occurred during ozonation of dissolved organic matter.47 The oxidation of the aromatic rings of GO via electrophilic addition reaction is consistent with the difference in carbon species distribution manifested by comparing the XPS results between GO-UV and GO-UV-nitrate (Table 1 and ESI† Fig. S11). For instance, the C–O content of GO-UV-nitrate (20.59%) was significantly higher than that of GO-UV (14.18%), which was probably due to the newly formed hydroxyl groups on GO. Interestingly, the O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O content of GO-UV-nitrate (4.96%) was significantly lower than that of GO-UV (10.68%), which was likely due to the decarboxylation of the carboxylic acids of GO. Fig. 7 illustrates these predominant reaction pathways of indirect photolysis by nitrate-induced ˙OH versus direct photolysis by photo induced electron–hole pairs.
O content of GO-UV-nitrate (4.96%) was significantly lower than that of GO-UV (10.68%), which was likely due to the decarboxylation of the carboxylic acids of GO. Fig. 7 illustrates these predominant reaction pathways of indirect photolysis by nitrate-induced ˙OH versus direct photolysis by photo induced electron–hole pairs.
4 Conclusions
Direct and indirect photolysis of GO lead to drastically different transformation products, which may exhibit dissimilar properties. Our study suggests that the significant effects of nitrate on GO photolysis were due to the indirect photolysis mediated by nitrate-induced ˙OH. Specifically, when reaching a concentration threshold of nitrate (e.g., 1 mM under the experimental conditions of the present study), the dominant reaction pathway of GO photolysis changed from direct photolysis to indirect photolysis. The concentration of nitrate is critical for determining the relative contributions of the direct vs. indirect photolysis pathways.The indirect photolysis pathway resulted in damage of the local π-conjugated structures, whereas direct photolysis resulted in restoration of π-conjugated structures. Notably, the indirect photolysis of GO mediated by ˙OH was not only driven by the oxidation reactions between ˙OH and oxidized domains, but also by the electrophilic addition reactions of ˙OH to the aromatic domains, causing substantial disintegration of GO nanostructures. The findings of this study underline the importance of indirect photolysis of GO in the presence of ROS precursors, and may have important implications for GO integrity and durability when used in water and wastewater treatment devices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was supported by the National Natural Science Foundation of China (Grants 41603099 and 21425729), Tianjin Municipal Science and Technology Commission (Grants 16JCYBJC22400, 17JCYBJC23100 and 17JCYBJC23200), the Ministry of Science and Technology of China (Grant 2014CB932001), the Fundamental Research Funds for the Central Universities and the 111 Program of Ministry of Education of China (T2017002). Partial funding was provided by the NSF ERC on Nanotechnology-Enabled Water Treatment (EEC-1449500).References
- X. Huang, Z. Yin, S. Wu, X. Qi, Q. He, Q. Zhang, Q. Yan, F. Boey and H. Zhang, Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications, Small, 2011, 7, 1876–1902 CrossRef CAS PubMed.
- Y. H. Ng, S. Ikeda, M. Matsumura and R. Amal, A perspective on fabricating carbon-based nanomaterials by photocatalysis and their applications, Energy Environ. Sci., 2012, 5, 9307–9318 RSC.
- H. Chang and H. Wu, Graphene-based nanocomposites: preparation, functionalization, and energy and environmental applications, Energy Environ. Sci., 2013, 6, 3483–3507 RSC.
- K. C. Kemp, H. Seema, M. Saleh, N. H. Le, K. Mahesh, V. Chandra and K. S. Kim, Environmental applications using graphene composites: water remediation and gas adsorption, Nanoscale, 2013, 5, 3149–3171 RSC.
- F. Perreault, A. Fonseca de Faria and M. Elimelech, Environmental applications of graphene-based nanomaterials, Chem. Soc. Rev., 2015, 44, 5861–5896 RSC.
- S. C. Smith and D. F. Rodrigues, Carbon-based nanomaterials for removal of chemical and biological contaminants from water: A review of mechanisms and applications, Carbon, 2015, 91, 122–143 CrossRef CAS.
- X. Zhu, K. Yang and B. Chen, Membranes prepared from graphene-based nanomaterials for sustainable applications: a review, Environ. Sci.: Nano, 2017, 4, 2267–2285 RSC.
- Z. Cai, A. D. Dwivedi, W.-N. Lee, X. Zhao, W. Liu, M. Sillanpaa, D. Zhao, C.-H. Huang and J. Fu, Application of nanotechnologies for removing pharmaceutically active compounds from water: development and future trends, Environ. Sci.: Nano, 2018, 5, 27–47 RSC.
- P. Westerhoff, P. Alvarez, Q. Li, J. Gardea-Torresdey and J. Zimmerman, Overcoming implementation barriers for nanotechnology in drinking water treatment, Environ. Sci.: Nano, 2016, 3, 1241–1253 RSC.
- Y. Shen, Q. Fang and B. Chen, Environmental Applications of Three-Dimensional Graphene-Based Macrostructures: Adsorption, Transformation, and Detection, Environ. Sci. Technol., 2015, 49, 67–84 CrossRef CAS PubMed.
- Y. Li, N. Yang, T. Du, X. Wang and W. Chen, Transformation of graphene oxide by chlorination and chloramination: Implications for environmental transport and fate, Water Res., 2016, 103, 416–423 CrossRef CAS PubMed.
- Y. Li, N. Yang, T. Du, T. Xia, C. Zhang and W. Chen, Chloramination of graphene oxide significantly affects its transport properties in saturated porous media, NanoImpact, 2016, 3–4, 90–95 CrossRef.
- X. Ren, J. Li, C. Chen, Y. Gao, D. Chen, M. Su, A. Alsaedi and T. Hayat, Graphene analogues in aquatic environments and porous media: dispersion, aggregation, deposition and transformation, Environ. Sci.: Nano, 2018, 5, 1298–1340 RSC.
- W.-C. Hou, I. Chowdhury, D. G. Goodwin, W. M. Henderson, D. H. Fairbrother, D. Bouchard and R. G. Zepp, Photochemical Transformation of Graphene Oxide in Sunlight, Environ. Sci. Technol., 2015, 49, 3435–3443 CrossRef CAS PubMed.
- H. Bai, W. Jiang, G. P. Kotchey, W. A. Saidi, B. J. Bythell, J. M. Jarvis, A. G. Marshall, R. A. S. Robinson and A. Star, Insight into the Mechanism of Graphene Oxide Degradation via the Photo-Fenton Reaction, J. Phys. Chem. C, 2014, 118, 10519–10529 CrossRef CAS PubMed.
- W. A. M. Hijnen, E. F. Beerendonk and G. J. Medema, Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review, Water Res., 2006, 40, 3–22 CrossRef CAS PubMed.
- H. Liu, S. Ryu, Z. Chen, M. L. Steigerwald, C. Nuckolls and L. E. Brus, Photochemical Reactivity of Graphene, J. Am. Chem. Soc., 2009, 131, 17099–17101 CrossRef CAS PubMed.
- O. Akhavan, M. Abdolahad, A. Esfandiar and M. Mohatashamifar, Photodegradation of Graphene Oxide Sheets by TiO2 Nanoparticles after a Photocatalytic Reduction, J. Phys. Chem. C, 2010, 114, 12955–12959 CrossRef CAS.
- Y. Matsumoto, M. Koinuma, S. Y. Kim, Y. Watanabe, T. Taniguchi, K. Hatakeyama, H. Tateishi and S. Ida, Simple Photoreduction of Graphene Oxide Nanosheet under Mild Conditions, ACS Appl. Mater. Interfaces, 2010, 2, 3461–3466 CrossRef CAS PubMed.
- Y. Matsumoto, M. Koinuma, S. Ida, S. Hayami, T. Taniguchi, K. Hatakeyama, H. Tateishi, Y. Watanabe and S. Amano, Photoreaction of Graphene Oxide Nanosheets in Water, J. Phys. Chem. C, 2011, 115, 19280–19286 CrossRef CAS.
- L. Guardia, S. Villar-Rodil, J. I. Paredes, R. Rozada, A. Martínez-Alonso and J. M. D. Tascón, UV light exposure of aqueous graphene oxide suspensions to promote their direct reduction, formation of graphene–metal nanoparticle hybrids and dye degradation, Carbon, 2012, 50, 1014–1024 CrossRef CAS.
- M. Koinuma, C. Ogata, Y. Kamei, K. Hatakeyama, H. Tateishi, Y. Watanabe, T. Taniguchi, K. Gezuhara, S. Hayami, A. Funatsu, M. Sakata, Y. Kuwahara, S. Kurihara and Y. Matsumoto, Photochemical Engineering of Graphene Oxide Nanosheets, J. Phys. Chem. C, 2012, 116, 19822–19827 CrossRef CAS.
- X. Zhou, Y. Zhang, C. Wang, X. Wu, Y. Yang, B. Zheng, H. Wu, S. Guo and J. Zhang, Photo-Fenton Reaction of Graphene Oxide: A New Strategy to Prepare Graphene Quantum Dots for DNA Cleavage, ACS Nano, 2012, 6, 6592–6599 CrossRef CAS PubMed.
- T. Ji, Y. Hua, M. Sun and N. Ma, The mechanism of the reaction of graphite oxide to reduced graphene oxide under ultraviolet irradiation, Carbon, 2013, 54, 412–418 CrossRef CAS.
- Y.-L. Zhang, L. Guo, H. Xia, Q.-D. Chen, J. Feng and H.-B. Sun, Photoreduction of Graphene Oxides: Methods, Properties, and Applications, Adv. Opt. Mater., 2014, 2, 10–28 CrossRef.
- C. Yu, B. Zhang, F. Yan, J. Zhao, J. Li, L. Li and J. Li, Engineering nano-porous graphene oxide by hydroxyl radicals, Carbon, 2016, 105, 291–296 CrossRef CAS.
- Y. Qiu, Z. Wang, A. C. E. Owens, I. Kulaots, Y. Chen, A. B. Kane and R. H. Hurt, Antioxidant chemistry of graphene-based materials and its role in oxidation protection technology, Nanoscale, 2014, 6, 11744–11755 RSC.
- A. Ianoul, T. Coleman and S. A. Asher, UV Resonance Raman Spectroscopic Detection of Nitrate and Nitrite in Wastewater Treatment Processes, Anal. Chem., 2002, 74, 1458–1461 CrossRef CAS.
- C. M. Sharpless and K. G. Linden, UV Photolysis of Nitrate:Effects of Natural Organic Matter and Dissolved Inorganic Carbon and Implications for UV Water Disinfection, Environ. Sci. Technol., 2001, 35, 2949–2955 CrossRef CAS PubMed.
- M. Zhan, X. Yang, Q. Xian and L. Kong, Photosensitized degradation of bisphenol A involving reactive oxygen species in the presence of humic substances, Chemosphere, 2006, 63, 378–386 CrossRef CAS PubMed.
- D. Zhang, S. Yan and W. Song, Photochemically Induced Formation of Reactive Oxygen Species (ROS) from Effluent Organic Matter, Environ. Sci. Technol., 2014, 48, 12645–12653 CrossRef CAS PubMed.
- P. L. Brezonik and J. Fulkerson-Brekken, Nitrate-Induced Photolysis in Natural Waters: Controls on Concentrations of Hydroxyl Radical Photo-Intermediates by Natural Scavenging Agents, Environ. Sci. Technol., 1998, 32, 3004–3010 CrossRef CAS.
- O. S. Keen, N. G. Love and K. G. Linden, The role of effluent nitrate in trace organic chemical oxidation during UV disinfection, Water Res., 2012, 46, 5224–5234 CrossRef CAS PubMed.
- R. C. Sandford, A. Exenberger and P. J. Worsfold, Nitrogen Cycling in Natural Waters using In Situ, Reagentless UV Spectrophotometry with Simultaneous Determination of Nitrate and Nitrite, Environ. Sci. Technol., 2007, 41, 8420–8425 CrossRef CAS PubMed.
- J. Mack and J. R. Bolton, Photochemistry of nitrite and nitrate in aqueous solution: a review, J. Photochem. Photobiol., A, 1999, 128, 1–13 CrossRef CAS.
- D.-H. Kim, J. Lee, J. Ryu, K. Kim and W. Choi, Arsenite Oxidation Initiated by the UV Photolysis of Nitrite and Nitrate, Environ. Sci. Technol., 2014, 48, 4030–4037 CrossRef CAS PubMed.
- F. Wang, F. Wang, G. Gao and W. Chen, Transformation of graphene oxide by ferrous iron: Environmental implications, Environ. Toxicol. Chem., 2015, 34, 1975–1982 CrossRef CAS PubMed.
- J. Lee, M. Cho, J. D. Fortner, J. B. Hughes and J.-H. Kim, Transformation of Aggregated C60 in the Aqueous Phase by UV Irradiation, Environ. Sci. Technol., 2009, 43, 4878–4883 CrossRef CAS PubMed.
- J. L. Bitter, J. Yang, S. Beigzadeh Milani, C. T. Jafvert and D. H. Fairbrother, Transformations of oxidized multiwalled carbon nanotubes exposed to UVC (254 nm) irradiation, Environ. Sci.: Nano, 2014, 1, 324–337 RSC.
- I. Chowdhury, W.-C. Hou, D. Goodwin, M. Henderson, R. G. Zepp and D. Bouchard, Sunlight affects aggregation and deposition of graphene oxide in the aquatic environment, Water Res., 2015, 78, 37–46 CrossRef CAS PubMed.
- Y. Zhao and C. T. Jafvert, Environmental photochemistry of single layered graphene oxide in water, Environ. Sci.: Nano, 2015, 2, 136–142 RSC.
- A. Ansón-Casaos, J. A. Puértolas, F. J. Pascual, J. Hernández-Ferrer, P. Castell, A. M. Benito, W. K. Maser and M. T. Martínez, The effect of gamma-irradiation on few-layered graphene materials, Appl. Surf. Sci., 2014, 301, 264–272 CrossRef.
- J. Lee, W. Song, S. S. Jang, J. D. Fortner, P. J. J. Alvarez, W. J. Cooper and J.-H. Kim, Stability of Water-Stable C60 Clusters to OH Radical Oxidation and Hydrated Electron Reduction, Environ. Sci. Technol., 2010, 44, 3786–3792 CrossRef CAS PubMed.
- S. Pei and H.-M. Cheng, The reduction of graphene oxide, Carbon, 2012, 50, 3210–3228 CrossRef CAS.
- S.-H. Hwang, D. Kang, R. S. Ruoff, H. S. Shin and Y.-B. Park, Poly(vinyl alcohol) Reinforced and Toughened with Poly(dopamine)-Treated Graphene Oxide, and Its Use for Humidity Sensing, ACS Nano, 2014, 8, 6739–6747 CrossRef CAS PubMed.
- S. Gligorovski, R. Strekowski, S. Barbati and D. Vione, Environmental Implications of Hydroxyl Radicals (˙OH), Chem. Rev., 2015, 115, 13051–13092 CrossRef CAS PubMed.
- J. Wenk, M. Aeschbacher, E. Salhi, S. Canonica, U. von Gunten and M. Sander, Chemical Oxidation of Dissolved Organic Matter by Chlorine Dioxide, Chlorine, And Ozone: Effects on Its Optical and Antioxidant Properties, Environ. Sci. Technol., 2013, 47, 11147–11156 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8en00637g |
| This journal is © The Royal Society of Chemistry 2019 |