Biomagnification of perfluoroalkyl acids (PFAAs) in the food web of an urban river: assessment of the trophic transfer of targeted and unknown precursors and implications†
Abstract
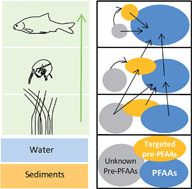
The present work examined the trophic transfer of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in a typical urban river (Orge River, near Paris, France), and aimed to investigate the potential contribution of precursors to the biomagnification of perfluoroalkyl acids (PFAAs). Sixteen PFAAs, twelve of their precursors (pre-PFAAstargeted) and two fluorinated alternatives to long-chain PFASs were analyzed in water, sediments and biota (including biofilm, invertebrates and fish). Twenty two compounds were detected in biological samples (2.0–147 ng g−1 wet weight), perfluorooctane sulfonate (PFOS) and C12–C14 perfluoroalkyl carboxylates (PFCAs) being predominant while ∑pre-PFAAstargeted contributed to 1–18% of ∑PFASs. Trophic magnification factors (TMFs) were >1 (i.e. denoting biomagnification) for C9–C14 PFCAs, C7–C10 perfluoroalkyl sulfonates (PFSAs) and several pre-PFAAs (e.g. 8 : 2 and 10 : 2 fluorotelomer sulfonates). The significant decrease in ∑pre-PFCAs/∑PFCAs concentration ratio with trophic level suggested a likely contribution of selected precursors to the biomagnification of PFCAs through biotransformation, while this was less obvious for PFOS. The total oxidizable precursor assay, applied for the first time to sediment and biota, revealed the presence of substantial proportions of extractable unknown pre-PFAAs in all samples (i.e. 15–80% of ∑PFASs upon oxidation). This proportion significantly decreased from sediments to invertebrates and fish, thereby pointing to the biotransformation of unattributed pre-PFAAs in the trophic web, which likely contributes to the biomagnification of some PFAAs (i.e. C9–C12 PFCAs and C7–C10 PFSAs).
- This article is part of the themed collections: PFAS and Best Papers 2019 – Environmental Science: Processes & Impacts


 Please wait while we load your content...
Please wait while we load your content...