Spatial and temporal variability of perfluoroalkyl substances in the Laurentian Great Lakes†
Abstract
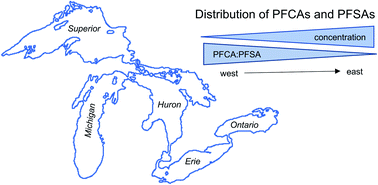
Per- and polyfluoroalkyl substances (PFAS) are a diverse group of fluorinated organic chemicals that have been used in industrial and consumer applications since the 1950s. PFAS are resistant to chemical and biological degradation and are ubiquitous in the environment, including in water, sediment, and biota in the Laurentian Great Lakes. This critical review evaluates the spatial and temporal variability of commonly studied perfluoroalkyl sulfonates (PFSAs) and perfluoroalkyl carboxylates (PFCAs) in the Great Lakes by synthesizing data collected in water, surface sediment, sediment cores, lake trout (Salvelinus namaycush), and herring gull (Larus argentatus) eggs. The lowest PFAS concentrations in all matrices are detected in Lake Superior, which is located in the most pristine region of the Great Lakes Basin. In contrast, higher concentrations are observed in Lakes Erie and Ontario, which are more impacted by industrial activity and wastewater discharge. The distribution of individual PFAS compounds also varies across the lakes in response to changes in PFAS sources, with higher proportions of PFSAs in the eastern lakes. Sediment and biota are enriched in long chain PFSAs and PFCAs relative to concentrations in the water column, as expected based on predicted partitioning behavior. Sediment cores and bioarchives consistently demonstrate that PFAS concentrations increased in the Great Lakes from the initial time points until the early 2000s. The available data indicate that PFOS and PFOA concentrations decline after this period in the upper Great Lakes, but are stable in Lake Ontario. However, these trends depend on the lake, the individual compound, and the organism considered.
- This article is part of the themed collection: PFAS


 Please wait while we load your content...
Please wait while we load your content...