Sulfamethoxazole degradation by an Fe(ii)-activated persulfate process: insight into the reactive sites, product identification and degradation pathways†
Abstract
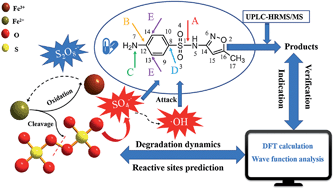
In this study, the effects of key parameters on the degradation kinetics of sulfamethoxazole (SMX) in an Fe(II)-activated persulfate (PS) process were elucidated. SMX could be completely degraded within 240 min at an initial pH of 3.3. It was found that 1 : 10 is the optimum molar ratio of Fe(II) : PS. Typical water quality parameters, including solution pH, SMX concentration, inorganic ions and humic acid, are discussed for the degradation process. Although the SMX degradation kinetics varied for different water quality parameters, relatively high SMX removal could always be achieved. The Fe(II)-activated persulfate process could maintain excellent SMX degradation under optimum reaction conditions. In addition, the reaction sites and intermediates of SMX were predicted by density functional theory (DFT) calculations and wave function analysis. The results of different calculations consistently indicate that N7 is the site with the highest electrophilic reactivity of SMX. The main intermediates formed were characterized through accurate mass measurement using UHPLC-HRMS/MS. Combined with the theoretical computations, the SMX degradation pathways in the Fe(II)-activated persulfate process are proposed. This research could provide theoretical guidance for the degradation mechanism of sulfonamides and provide technical support for the design of efficient degradation reactions in the future.
- This article is part of the themed collection: Contaminant remediation and fate


 Please wait while we load your content...
Please wait while we load your content...