Previously unidentified sources of perfluoroalkyl and polyfluoroalkyl substances from building materials and industrial fabrics†
Abstract
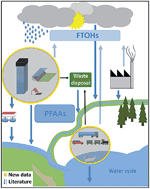
Perfluoroalkyl and polyfluoroalkyl substances (PFASs) are applied during the production of various consumer and industrial goods. As a consequence of their use in building materials and fabrics, unreacted nonpolymeric PFASs might enter the environment by evaporation or urban run-off. Since the PFAS content of building materials and industrial fabrics is hardly investigated, studies have to be performed in order to assess their total PFAS load. Building material samples (n = 23) and fabric samples (n = 28) were collected and their PFAS content was investigated. A total of 29 PFASs were analyzed (chain length in the range of C4–C14). PFASs of diverse chain lengths were detected in 53% of the analyzed samples. The sum of PFASs for awning materials and coating samples were amongst the highest. Furthermore, PFASs were detected in the majority of fabrics for maritime applications, public transport seat covers and fluoropolymer facade materials. To the best of our knowledge, this study was the first to investigate the PFAS concentrations in fabrics for maritime applications, fluoropolymer facade materials and coatings for architectural purposes. Thus, new sources of PFASs were identified that might lead to release of PFASs into the environment.
- This article is part of the themed collection: PFAS


 Please wait while we load your content...
Please wait while we load your content...