Open Access Article
Open Access ArticleThe behaviour of irrigation induced Se in the groundwater-soil-plant system in Punjab, India†
Elisabeth
Eiche
 *a,
Alexandra Kelly
Nothstein
*a,
Alexandra Kelly
Nothstein
 b,
Jörg
Göttlicher
c,
Ralph
Steininger
c,
Karaj Singh
Dhillon
d and
Thomas
Neumann
e
b,
Jörg
Göttlicher
c,
Ralph
Steininger
c,
Karaj Singh
Dhillon
d and
Thomas
Neumann
e
aInstitute of Applied Geosciences, Karlsruhe Institute of Technology (KIT), Adenauerring 20b, 76131 Karlsruhe, Germany. E-mail: elisabeth.eiche@kit.edu
bSUM, KIT, Hermann-von-Helmholtz-Platz 1, D-76344 Eggenstein-Leopoldshafen, Germany
cInstitute of Photon Science & Synchrotron Radiation, KIT, Hermann-von-Helmholtz-Platz 1, D-76344 Eggenstein-Leopoldshafen, Germany
dDepartment of Soil Science, Punjab Agricultural University, Ludhiana 141004, India
eInstitute of Applied Geosciences, Technische Universität Berlin, Ernst-Reuter-Platz 1, 10587 Berlin, Germany
First published on 15th April 2019
Abstract
Selenium is of special interest in different research fields due to its narrow range between beneficial and toxic effects. On a global scale, Se deficiency is more widespread. Biofortification measures have successfully been applied to specifically increase Se concentrations in food crops. Still not much is known about the behaviour and long-term fate of externally supplied Se. Over many years, natural but external selenate is regularly introduced into the soil-plant system via irrigation at our study sites in Punjab which makes it also an ideal natural analogue to investigate the long term effect of biofortification. For our study, we combined total and species specific analysis of Se in soil and plant material. Selenium is clearly enriched in all investigated topsoils (0–15 cm) with concentrations of 1.5–13.0 mg kg−1 despite similar background Se concentrations (0.5 ± 0.1 mg kg−1) below 15 cm depth. Irrigation is indicated to be the primary source of excess Se. Processes like Se species transformation, uptake by plants and plant material decomposition further influence both the Se speciation and extent of Se enrichment in the soils. The Se concentration in different plants and plant parts is alarmingly high showing concentrations of up to 738 mg kg−1 in wheat. Irrigation induced selenate can be considered as an easily available short term pool of Se for plants and thus strongly controls their total Se concentration and speciation. The long-term pool of Se in the topsoil mainly consists of selenite and organic Se species. These species are readily retained but still sufficiently mobile to be taken up by plants. The formation of elemental Se can be considered as a non-available Se pool and is thus, the major cause of Se immobilization and long-term enrichment of Se in the soils. Our study clearly shows that biofortification with selenate, despite its effectiveness, bears the risk of easily increasing Se levels in plants to toxic levels and producing food with less favourable inorganic Se species if not done with care. Excess selenate is either lost due to biomethylation or immobilized within the soil which has to be considered as highly negative from both an economic and ecological point of few.
Environmental significanceSelenium is often artificially supplemented to agricultural systems due to its essentiality for humans despite a lack of knowledge of the long-term behaviour of this also toxic element. Investigations on the fate of irrigation induced selenate clearly proved an alarmingly high enrichment of Se in both soil and plants. In soils, the enrichment is largely controlled by the formation of immobile elemental Se making soils to a long-term Se pool. In plants, the excess of Se leads to a dominance of inorganic Se species which are less favourable from a food and health perspective. Thus, Se species transformation is a key process that has to be understood when supplying artificial Se to natural systems without causing long-term system disturbance. |
Introduction
Selenium (Se) is a micronutrient which is often called a “double-edged sword”1 due to its ambivalent nature with regard to human and animal health. On the one hand, Se is an essential trace element with multiple positive functions like reducing the risk of cancer and cardiovascular diseases or enhancing the detoxification of heavy metal intake.2–4 On the other hand, both low and excessive dietary intake could cause various health risks or disorders in humans and livestock.5–7 The range of adequate Se intake is one of the narrowest known.8,9 Recommended limits of safe daily dietary Se intake typically vary between 55 and 400 μg d−1 for adult humans8,10 but the onset of actual pharmacological and toxicological effects further depend on age, gender and weight of the individual person.2,11,12 For livestock, 0.05–0.1 mg Se per kg dry weight (dw) are suggested as minimum requirement in their feed while toxic effects are expectable above 2–5 mg Se per kg dw.13,14 The actual impact, however, varies with animal species.15The bioavailability and bioaccessibility and thus, also its health effect, are further controlled by the form of ingested Se.4,16,17 Plants, which are the main Se source for humans and animals,5,6,18,19 play a decisive role in this context due to their ability to transform inorganic species that are taken up from the soil into organic Se species that are mainly less toxic and more accessible.19
High Se concentrations in water can be of concern if transferred to the soil-plant system via irrigation leading to toxic Se concentrations in food as reported in several cases worldwide.20–22 Therefore, limits for Se in irrigation water of 10–20 μg L−1 were advised by several states and organisations in the past.23–26
Selenium concentrations in soils are generally low with 0.01–2 mg kg−1 (average: 0.4 mg kg−1) but soils with highly elevated Se concentrations (>2–5000 mg kg−1), called seleniferous soils, are widely distributed throughout the world (USA: ≤28 mg kg−1; Ireland: ≤1200 mg kg−1, China: ≤59 mg kg−1).1,26–28 In India, both Se deficient (0.025–0.71 mg kg−1) and seleniferous soils (1–20 mg kg−1) have been described.26 Primarily, the Se concentration in soils is determined by its content in the parent rock as well as the topography and climate. Over time, however, deposition of seleniferous erosion material, poor drainage of soils, irrigation with Se containing water and input through mining operations, volcanic eruptions, precipitation, combustion of coal or petroleum can considerably overprint the background Se content in soils.26,28–31 Consequently, the Se distribution is usually heterogeneous and site specific.32,33
It is generally acknowledged that Se transfer from soils into plants and thus into the food chain is controlled by the Se speciation rather than its total concentration.11,33 Selenate is seen as the most mobile and bioavailable form in soils because of its weak adsorption to minerals through electrostatic forces and its inability to form insoluble salts.33,34 The lower mobility and availability of selenite to plants can be explained by its high affinity to clay minerals and Al/Fe oxyhydroxides to which it is fixed through strong inner-sphere surface complexes.35,36 Both inorganic species can be transformed by biotic and abiotic processes but typically co-exist in soils.37,38 Under anoxic conditions, microbes are able to further reduce selenite to insoluble elemental Se or selenides.39–42 Methylated Se species, which are often volatile, are another product of microbial processes43 and can be an important factor for the loss of Se from the soils directly to the atmosphere.44 In general, the behaviour of organic Se species in the soil-plant system is less clear but organic Se species have been reported to be readily taken up by plants.45–47 Selenium bound to organic matter, however, is considered as important long-term pool for Se in soils because of its low direct bioavailability. In short, the Se speciation and thus its bioavailability is susceptible to various reactions and processes in soil such as redox-transformations and pH shifts, adsorption/desorption, precipitation/dissolution, Se–ligand complex formation and methylation.48
Plants differ in their ability to accumulate Se in their tissue. A distinction is made between primary accumulator (1000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg per kg dry weight (dw)), secondary accumulator (<1000 mg per kg dw) and non-accumulator plants which mainly contain less than 50 mg per kg dw and often even less than 5 mg kg−1.49,50 Most forage and crop plants belong to the non-accumulator plants with rarely more than 25 mg Se per kg dw if grown on seleniferous soils51 and 0.01–1 mg Se per kg on non-seleniferous soils.52,53 Unfortunately, no generally applicable threshold value can be formulated as precaution to prevent toxicity symptoms in plants and thus economic losses e.g. due to reduced yield for farmers. This is because the extent of both beneficial and toxic effects of Se on plants is determined by several factors like Se concentration and speciation, plant species or agricultural practices.38,51
000 mg per kg dry weight (dw)), secondary accumulator (<1000 mg per kg dw) and non-accumulator plants which mainly contain less than 50 mg per kg dw and often even less than 5 mg kg−1.49,50 Most forage and crop plants belong to the non-accumulator plants with rarely more than 25 mg Se per kg dw if grown on seleniferous soils51 and 0.01–1 mg Se per kg on non-seleniferous soils.52,53 Unfortunately, no generally applicable threshold value can be formulated as precaution to prevent toxicity symptoms in plants and thus economic losses e.g. due to reduced yield for farmers. This is because the extent of both beneficial and toxic effects of Se on plants is determined by several factors like Se concentration and speciation, plant species or agricultural practices.38,51
Due to its significant role with regard to human and animal health it is generally acknowledged that ensuring an adequate Se intake in both deficient and seleniferous areas is highly important. So far, however, the Se status of a region is mainly determined based on total Se contents in soil. The complexity of processes and interactions that control the transfer of Se into plants highly questions this approach. Different concepts have been developed over the years to intentionally increase or decrease the Se content in food. Many biofortification measures aim at increasing the concentration of bioavailable Se in soils. In this context, still not enough attention is paid to the influence of Se speciation in soil on plant and food Se speciation and their relation to health.54 To develop successful Se management concepts that are cost- and resource-efficient, the understanding of all interacting processes that determine the Se mobility, transfer and speciation in the soil-plant system has to be significantly improved. Studies in controlled environments are helpful to elucidate individual processes but often oversimplify important chemical, physical and biological processes that are present in nature.33 The aim of this paper is to study the relation between Se concentration and speciation in groundwater, soil and different plant species in the context of long-term enrichment and toxicity in a known seleniferous area in Punjab, India, under natural conditions.
Study area
The study area, a small seleniferous area (∼1000 ha) first described by Dhillon and Dhillon,55 is located in the state of Punjab, North-West India. It stretches over the districts of SBS Nagar (formerly known as Nawanshahr, 33.12°N, 76.13°E) and Hoshiarpur (31.12°N 76.13°E) and lies at the foothills (Shivalik range) of the Himalaya. All samples were taken near the villages of Jainpur and Barwa (Fig. S1†). Around both villages, approximately 400 ha are described as seleniferous (soil Se content >0.5 mg kg−1) and 200 ha as highly seleniferous (>2 mg kg−1).56,57The area has a humid subtropical climate with hot summers and cold winters (6–45 °C).58 Approximately 80% of the rainfall occurs during the monsoon from June to September. Most soils of the area are used for agriculture with wheat, maize, rice, sugarcane and forage crops (oat, clover, mustard etc.) as dominant cultivars. Nowadays, wheat-rice rotation (70%) is the dominant form of land use, which has largely replaced the former wheat-maize rotation.59 Sugarcane is grown on about 20% of the cultivated area.59 The growth period of wheat is from October to March/April. Most fields have to be regularly irrigated mainly using groundwater – the only source of water for irrigation due to extended periods of dryness especially during the winter and the high water demand of rice cultivation.21 The demand of water for rice-wheat rotation (∼2000 L m−2 a−1) is 3.3 times higher compared to maize-wheat rotation (∼600 L m−2 a−1).59,60
Crops grown in the area, especially wheat, regularly show toxicity symptoms like white and pink chlorosis. Furthermore, signs of selenosis have been reported from the area both in human and animals.21,55,61 The daily dietary intake by humans clearly differs between seleniferous areas with 250–1150 μg per day per day and non-seleniferous areas with 40–80 μg per day.62 However, over the last years, no incident of selenosis of humans has been reported which might be due to a changed diet with a greater share of globally produced food; due to awareness about farm produce or an adaption to elevated Se concentrations.63
Materials and methods
Soil and plant sampling
Soil and plant samples were taken near the villages of Jainpur and Barwa (Fig. S1†). The sampling sites were selected based on the known distribution of seleniferous soils and visible signs of chlorosis to also include extreme sites into our study. Three soil depth profiles (Field-1, Field-2, Field-3) (Fig. S1†) divided into 0–2, 2–5, 5–10, 10–15, 15–30, 30–45 and <45 cm were sampled where wheat was growing during that time (Fig. S2†). The samples were taken by digging a small pit. For each depth interval, the respective layer was sampled horizontally starting with the top 2 cm. Samples > 45 cm were taken with a soil auger. Where plant samples were taken, a bulk soil sample of the root zone, which equals the top 15 cm, was taken. Field-1, located 1 km east of Barwa village, was situated next to an irrigation channel (Fig. S2†), which was dry during sampling time. Field-2 and Field-3 were located 1.5 km north-west of Field 1. The samples were taken approximately one meter inside the respective field. Additionally, wheat plants (Triticum aestivum) were collected at each site. The wheat plants at Field-1 showed signs of white chlorosis (Fig. S2†), which was not visible at the other sites. Additionally, plant samples of Indian mustard (Brassica juncea), sugarcane (Saccharum officinarum), clover (Trifolium spec.), garlic (Allium sativum) and wheat (Triticum aestivum) showing pink chlorosis (Fig. S2†) were taken. All samples were air dried in the laboratory of the Department of Soil Science of the Punjab Agricultural University.Soil and plant bulk analysis
The bulk elemental composition of all soil samples was determined with energy dispersive X-ray fluorescence analysis (ED XRF, Epsilon 5, PANalytical) using bulk powder samples in spectro cups, sealed with a Mylar film of six μm thickness. A tungsten X-ray tube was used as radiation source, whereas a Ge-detector was used for detection and quantification. In order to optimize the fluorescence measurements, each sample was analysed by consecutively using BRAKLA – polarization targets (Al2O3) and secondary targets (CaF2, Fe, Ge, KBr, Zr, Mo, Ag, CsI). A matrix specific calibration using standard addition was made for Se by spiking original soil material with different concentrations of a dissolved Se (Merck calibration standard) to lower the Se detection limit to ∼0.5 mg kg−1. Selenium was determined using excitation by the Zr-secondary target, running the W-tube at 80 kV/6 mA for a measuring time of 500 s per sample. The accuracy was tested by including the reference material GRX5 (Park City, Utah, USA) into the measuring protocol showing a slight overestimation of Se of 10%. The accuracy of all other elements is given in the ESI.†To check for the quality of the ED XRF analysis, especially of the samples with Se concentrations below 1 mg kg−1, Se was also determined by ICP-MS (X-Series2, Thermo Scientific) after a microwave acid digestion using HNO3 (65%, subboiled), H2O2 (30%, p.a.) and HF (40%, suprapur). 100 mg of pulverized sample material was used for each sample and standard. The accuracy of the ICP-MS analysis (Se ≤ 6.8%) was tested by including the certified standard HPS CRM-TMDW (High Purity Standards, USA) into the measurements. The quality of the digestion was tested by including the GXR4 and GRX5 reference material (Park City, Utah, USA) into the digestion process. The Se concentration of GXR5 was slightly underestimated by <12% probably due to losses of volatile Se. The Se concentration given in this study is based on ICP-MS measurements after correction of the underestimated proportion (see in the ESI† for more details). To check for the reproducibility of the results, the samples from Field-1 were all digested and measured in duplicate (Table S1†). More details on the results of the quality measures are given in the ESI.†
The content of organic carbon (Corg) was determined after decarbonization using a Carbon–Sulfur-Analyser (CS 2000, Eltra). A cement reference standard (90811-13) was used to check both accuracy (100 ± 1%) and reproducibility (±0.8%, n = 5). The pH-value of selected soil samples was determined both in H2O and 0.01 M CaCl2 suspension with a WTW pH-sensor (SenTix 81). More details on the method and results of the quality measures are given in the ESI.†
Air dried plants were separated into different plant parts (root, stem, leaves, head, flower), freeze-dried for 24 h and grinded to powder or cut into small pieces. The Se concentration of the plant material was determined using ICP-MS analysis (X-Serie2, Thermo Scientific) after microwave digestion (Start 1500, MLS). One mL Milli-Q water, 5 mL HNO3 (65%, subboiled) and 1 mL H2O2 (30%, p.a.) was used for 200 mg of each sample. Plant material from seven different sites was digested. If available, plant material from two different plants from the same site were digested also which adds up to 28 samples. For quality assurance of the digestion process, three samples were analysed in duplicate. The difference in Setot was ≤4.5% (Table S7†). The accuracy of the ICP-MS analysis (Se ≤ 8%) was tested by including the certified standard HPS CRM-TMDW (High Purity Standards, USA) into the measurements. For more details on the sample digestion and results of quality measures, please refer to the ESI.†
Sequential extraction and XANES analysis
Information about the Se speciation and way of fixation was gained using the sequential extraction scheme of Zhang and Moore64 which was modified according to Wright et al.65 In the protocol, six operationally defined Se fractions are distinguished: Fraction 1: easily soluble Se (using 0.25 M KCl), Fraction 2: adsorbed Se (0.1 M K2HPO4), Fraction 3: elemental Se (0.25 M Na2SO3), Fraction 4: organically associated Se (5% NaOCl), Fraction 5: Se associated with amorphous oxides and carbonates (4 M HCl) and Fraction 6: residual Se (HNO3, H2O2, HF microwave digestion). Details on the limitations of sequential extractions are discussed in Wright et al.65 and Bacon & Davidson.66 Two gram (air dried) of all root zone soil samples as well as soil from 0–2 cm, 2–5 cm and 5–10 cm of Field-1 to -3 were subjected to sequential extraction. The concentration of Se in each fraction was measured by ICP-MS (X-Series2, Thermo Scientific). The accuracy of the ICP-MS analysis (Se ≤ 6%) was tested by including the certified standard HPS CRM-TMDW (High Purity Standards, USA) into the measurement protocol. The quality of the extraction was checked by including the GXR4 reference material (Park City, Utah, USA) into the extraction procedure (Se: 97, 106%). Additionally, two samples (Mustard root zone, Field-1, 0–2 cm) were extracted in duplicate and one sample in triplicate (Field-2, 2–5 cm). The total Se concentration differed less than 1% for the samples analysed in duplicate and less than 10.1% for the one analysed in triplicate (Table S5†). For more details on the quality assurance of the sequential extraction, please refer to the ESI.†The relative binding intensity of Se within the different soils was characterized using the reduced partition index (IR)67–69 that is based on the results of the sequential extraction. A low value of IR is indicative of a high proportion of soluble Se whereas high values (close to 1) stand for a dominance of Se that is bound in the residual fraction.
The Se speciation in bulk soil, single particles and in bulk material of different plant parts was measured on pressed pellets of ground material and unsupported thin sections (single particles) using X-ray absorption spectroscopy (Fig. S4†). The experiments were carried out at the SUL-X beamline of the synchrotron radiation source of the Karlsruhe Institute of Technology (KIT). Selenium K-edge spectra were recorded using a monochromator with Si(111) crystals. The beam spot size was 1 × 1 mm2 for bulk and 0.1 × 0.1 mm2 for single particle analysis. The beam spot size was 1 × 1 mm2 for bulk and 0.1 × 0.1 mm2 for single particle analysis. The photon flux density was 1.5 × 1011 photons per s. XAS measurements were performed in fluorescence mode (Se Kα X-ray fluorescence) using solid state detectors (one element Vortex silicon drift detector (SDD)). The XAS scan energy ranged from ∼200 eV below the first inflection point of elemental Se (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 658 eV) to ∼12
658 eV) to ∼12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 eV. Short scan times of ∼10 min were chosen in order to avoid beam-induced redox reactions or volatilization of organic Se species. Each spectrum is the merge of two to six measurements at slightly different spots (Fig. S5†). A spectrum of elemental Se (8 wt%) was collected simultaneously with each scan for energy calibration. The identification of the individual Se species and its respective contribution to the measured spectra was calculated by linear combination fitting (LCF) in the range of −20 to +120 eV using several Se references and the IFEFFIT software package.70 Elemental Se (Se0), selenate (SeVI, Na2SeO4x10H2O) and selenite (SeIV, Na2SeO3) were used in a diluted form as inorganic references. The spectra of organic Se species included secysteine (SeCys), semethionine (SeMet), dimethyl-selenide (DMeSe), Se-methyl-selenoL-cysteine (SeMeCys). Apart from SeMet, all organic spectra were kindly provided by G. Sarret.
900 eV. Short scan times of ∼10 min were chosen in order to avoid beam-induced redox reactions or volatilization of organic Se species. Each spectrum is the merge of two to six measurements at slightly different spots (Fig. S5†). A spectrum of elemental Se (8 wt%) was collected simultaneously with each scan for energy calibration. The identification of the individual Se species and its respective contribution to the measured spectra was calculated by linear combination fitting (LCF) in the range of −20 to +120 eV using several Se references and the IFEFFIT software package.70 Elemental Se (Se0), selenate (SeVI, Na2SeO4x10H2O) and selenite (SeIV, Na2SeO3) were used in a diluted form as inorganic references. The spectra of organic Se species included secysteine (SeCys), semethionine (SeMet), dimethyl-selenide (DMeSe), Se-methyl-selenoL-cysteine (SeMeCys). Apart from SeMet, all organic spectra were kindly provided by G. Sarret.
Statistical data analysis
Pearson correlation coefficients (rP) were calculated to determine and confirm relationships between different elements or parameters using the software package Originlab2015. Given errors always refer to the first standard deviation.Results
Soil geochemistry
The Setot concentration is in the range of <1 mg kg−1 in the lower layers of all soil profiles up to 13.0 mg kg−1 in the top soil of Field-1. Generally, the highest Se concentrations are detectable in the upper 15 cm of all soil profiles with maximum values in a depth between 2 and 5 cm (Fig. 1 and Table S1†). The Setot concentrations in the root zone soil of mustard, sugarcane, garlic, clover and the pink wheat are 6.1, 4.7, 2.0, 3.3 and 3.5 mg kg−1 (Table 1). The depth distribution of CaO, Sr and Corg are similar to Setot with highest concentrations in the upper 15 cm in all soil profiles (Fig. 1, Table S2†). The Corg content is ≤0.43 wt% in the lower layers and up to 1.01 wt% in the top soil of Field-1 (Table S4†). Strontium concentrations are in the range of 101–116 mg kg−1 in the layers >15 cm and from 110–158 mg kg−1 in the top 15 cm. Calcium is enriched in the top layers with up to 4.6 wt% CaO and only 1.2–3 wt% CaO in a depth > 30 cm (Table S2†). All soils are slightly alkaline with pH values of 7.5–8.1 (CaCl2) and 8.5–9.1 (H2O) (Table 1), respectively.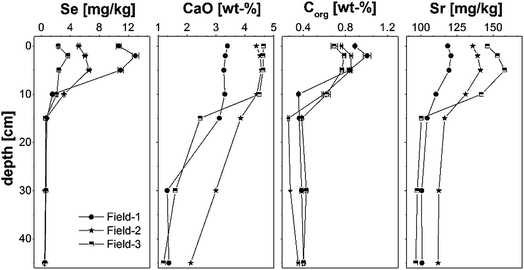 | ||
| Fig. 1 Depth distribution of Se, CaO, Corg and Sr concentration in the soil profile at the Fields-1 to -3. The errors given stand for the maximum standard deviation of duplicate measurements. | ||
| Field-1 | Field-2 | Field-3 | Pinkish wheat | Sugarcane | Garlic | Clover | Indian mustard | |
|---|---|---|---|---|---|---|---|---|
| Topsoil Setot [mg kg−1] | 9.0 | 5.2 | 2.6 | 3.5 | 4.7 | 2.0 | 3.3 | 6.1 |
| I R | 0.33 | 0.41 | 0.49 | 0.37 | 0.44 | 0.48 | 0.42 | 0.43 |
| pH-value (in H2O) | 8.6 | 8.7 | 8.6 | 9.1 | 8.5 | 8.7 | — | 8.5 |
| pH-value (in CaCl2) | 7.8 | 7.8 | 7.8 | 8.1 | 7.5 | 7.8 | — | 7.6 |
Selenium speciation in soil
Considering all soil samples, the fractions that are associated with elemental Se and oxidically/carbonate bound Se are dominant with a proportion of 23–58% and 14–33%, respectively. Selenium associated with organic material also has a considerable relevance in most of the samples with 9–18%. Easily available, adsorbed and residual Se have a proportion of 5–22%, 4–9% and 4–11%, respectively (Table 2).| Selenium fraction | Field-1a | Field-2 | Field-3 | Pink wheat soil | Mustard soila | Garlic | Sugarcane soil | Clover soil | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | mg kg−1 | % | mg kg−1 | % | mg kg−1 | % | mg kg−1 | % | mg kg−1 | % | mg kg−1 | % | mg kg−1 | % | mg kg−1 | |
| a Data published in Eiche et al.84 | ||||||||||||||||
| Easily available | 10 | 1.2 | 10 | 0.7 | 8 | 0.2 | 22 | 0.8 | 7 | 0.5 | 5 | 0.1 | 5 | 0.3 | 14 | 0.6 |
| Adsorbed | 6 | 0.7 | 6 | 0.4 | 8 | 0.3 | 6 | 0.2 | 9 | 0.6 | 5 | 0.1 | 6 | 0.4 | 4 | 0.2 |
| Elemental |
|
7.1 ± 0.5 |
|
2.6 | 23 | 0.7 |
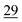
|
1.1 |
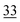
|
2.3 | 30 | 0.7 |
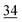
|
1.9 |
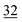
|
1.4 |
| Organically associated | 9 | 1.1 | 15 | 1.0 | 12 | 0.4 | 14 | 0.6 | 16 | 1.1 | 18 | 0.4 | 18 | 1.0 | 18 | 0.8 |
| Oxidically/carbonate bound | 14 | 1.7 | 19 | 1.3 |
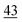
|
1.3 | 25 | 1.0 | 28 | 1.9 |
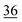
|
0.8 |
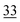
|
1.8 | 22 | 0.9 |
| Residual | 4 | 0.5 | 11 | 0.7 | 7 | 0.2 | 5 | 0.2 | 7 | 0.4 | 6 | 0.2 | 4 | 0.2 | 10 | 0.4 |
The share of Se in the individual fractions is more or less similar in the samples of 0–2, 2–5, 5–10 cm depth at Field-1 to Field-3 (Table S6†). Consequently, the mean of the three depth will be considered for each fraction throughout this paper. The given error stands for the standard deviation of the three depth. Field-1 soil has the highest proportion of Se in the elemental fraction (58 ± 1.9%) and at the same time the lowest proportion of Se in the oxidically/carbonate bound fraction (14 ± 1.1%) of all samples. Selenium in the mobile fractions adds up to 16.1 ± 2.1% from which 10.0 ± 0.6% are in the easily soluble fraction and 6 ± 0.5% are indicated to be adsorbed at Field-1. The presence of elemental Se as well as selenite was also clearly indicated by XAS analysis at Field-1 with a share of 80 and 20%, respectively in the single particle analysis (Fig. 2). In the bulk sample, also organic Se was identified with 20% in addition to elemental Se (30%) and selenite (50%). At Field-2, elemental Se (39 ± 3.1%) is again the main Se fraction but also Se in the organically associated (15 ± 0.2%) and oxidically/carbonate bound fraction (19 ± 0.3%) are present in a relatively high proportion. The percentage of Se in the easily available and adsorbed fraction is nearly similar to Field-1 with 10 ± 1.4% and 6 ± 0.4%, respectively. The opposite trend is detectable at Field-3 where the oxidically/carbonate bound fraction is dominant with 43 ± 4.3% and only 23 ± 0.7% in the elemental fraction. Again, the sum of the mobile fractions is comparable to the two other sites with 16 ± 0.5%.
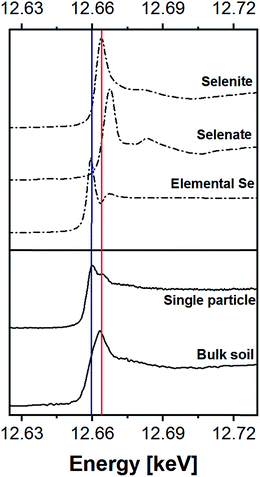 | ||
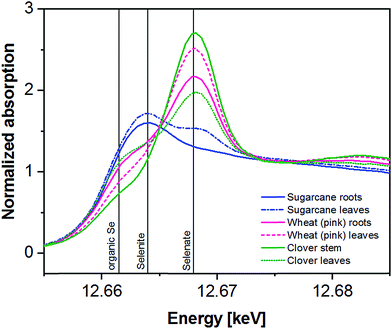
| Fig. 2 XANES spectra of Se references in comparison to the spectra of a bulk soil sample and a single particle measurement of Field-1. | ||
The root zone soil of the wheat showing pink chlorosis has by far the highest proportion (22%) of Se in the easily available fraction. The percentage of Se in the elemental and oxidically/carbonate bound fraction is nearly even with 29 and 25%, respectively. The root zone soils of Indian mustard, sugarcane and garlic are relatively similar with the same proportion of Se in the elemental (30–34%) and oxidically/carbonate bound fraction (28–36%). In these soils, the percentage of Se in the easily available fraction is very low with 7 and 5%, respectively. However, the actual concentration in the garlic soil in each fraction is much lower due the lower Setot (Table 1). In the clover root zone soil, 14% of the Se is in the easy available fraction. Again, the dominant Se fraction is elemental Se (32%) followed by oxidically/carbonate bound Se (22%) and organically associated Se (18%).
Selenium concentration in plants
The Se concentration is generally very high in all plant parts with a maximum of 931 mg kg−1 in the Indian mustard leaves. However, the concentrations vary considerably between the plant parts of the same species and especially between the different plant species (Tables 3 and S8†). Still there is an increasing trend in Se concentration towards the top of most of the plants.| Plant parts [mg kg−1] | Wheat | Sugarcane | Garlic | Clover | Indian mustarda | |||
|---|---|---|---|---|---|---|---|---|
| Field-1a | Field-2 | Field-3 | Pink | |||||
| a Data published in Eiche et al.84 | ||||||||
| Roots | 196 | 47 | 6.0 | 192 | 56 | 41 | 49 | 186 |
| Stem | 191 | 29 | 1.3 | 146 | 57 | — | 61 | 130 |
| Leaves | 387 | 36 | 6.8 | 738 | 86 | 28 | 55 | 931 |
| Flower | — | — | — | — | — | — | 64 | 541 |
| Head | — | 36 | 3.2 | — | — | — | — | — |
| Bulb | — | — | — | — | — | 14 | — | — |
| Leave/root ratio | 2.0 | 0.8 | 1.1 | 3.8 | 1.5 | 0.7 | 1.1 | 5.0 |
In total, the highest Se concentrations can be found in Indian mustard, a semi-accumulator plant,19 ranging from 186 mg kg−1 in the root up to 931 mg kg−1 in the leaves. In our study, garlic has much lower Se concentrations compared to Indian mustard despite being a known accumulator of Se.49 Here, highest concentrations were found in the roots with 41 mg kg−1 and lowest in the bulb with 14 mg kg−1. From the non-accumulator plants, highest Se concentrations were found in the wheat showing pink chlorosis with up to 738 mg kg−1 in the leaves and 182 mg kg−1 in the roots. The Se concentration in the wheat at Field-1, which shows white chlorosis, is also extremely high with 196 mg kg−1 in the roots and up to 387 mg kg−1 in the leaves. At the other two sites, where no chlorosis was detectable, Se concentrations in wheat are considerably lower but still high with 29 (stem) to 47 mg kg−1 (roots) at Field-2 and 1.3 (stem) to 6.8 mg kg−1 (leaves) at Field-3. The Se concentration in clover shows no large variations with the highest enrichment in the flower (64 mg kg−1) and lowest in the roots (49 mg kg−1).
Selenium speciation in plants
In general, all investigated plant parts show distinct peaks or shoulders at approx. 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 662 or 12.664 eV which is a typical energy for organic Se species or selenite in the latter case. The peak at approx. 12
662 or 12.664 eV which is a typical energy for organic Se species or selenite in the latter case. The peak at approx. 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 668 eV is clearly indicative of selenate (Fig. 3). The quantification of organic Se and inorganic Se species and the separation of selenate and selenite was done using LCF (Fig. S7†). Due to the difficulty to clearly distinguish between individual organic Se species, only the total of fitted organic species is considered within this study.
668 eV is clearly indicative of selenate (Fig. 3). The quantification of organic Se and inorganic Se species and the separation of selenate and selenite was done using LCF (Fig. S7†). Due to the difficulty to clearly distinguish between individual organic Se species, only the total of fitted organic species is considered within this study.
The XAS-spectra of sugarcane roots (Fig. 3) are dominated by one clearly visible peak at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 663.8 eV, which can be fitted using organic Se species (80%) and selenite (20%). In the spectra of the upper leaves, two peaks at 12
663.8 eV, which can be fitted using organic Se species (80%) and selenite (20%). In the spectra of the upper leaves, two peaks at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 663.8 (organic Se, selenite) and 12
663.8 (organic Se, selenite) and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 668.1 eV (selenate) can clearly be identified LCF resulted in 63% organic and 37% inorganic Se (with selenite as dominating species) (Table 4).
668.1 eV (selenate) can clearly be identified LCF resulted in 63% organic and 37% inorganic Se (with selenite as dominating species) (Table 4).
| Selenite | Selenate | Seinorg | Seorg | |
|---|---|---|---|---|
| a Published in Eiche et al.84 (added for better comparison). | ||||
| Wheat (S1 ) | ||||
| Roots | 12 | 13 | 25 | 75 |
| Stem | 11 | 18 | 29 | 71 |
| Leaves | 10 | 47 | 57 | 43 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Wheat (pink) | ||||
| Roots | 22 | 34 | 56 | 44 |
| Leaves | 24 | 51 | 75 | 25 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Sugarcane | ||||
| Main roots | 20 | — | 20 | 70 |
| Upper leaves | 30 | 7 | 37 | 63 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Clover | ||||
| Stem | 17 | 64 | 81 | 19 |
| Leaves | 15 | 27 | 42 | 58 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Mustard | ||||
| Roots | 16 | 28 | 44 | 56 |
| Stem | 10 | 52 | 62 | 38 |
| Leaves | 6 | 70 | 76 | 34 |
Wheat showing pink chlorosis also is clearly dominated by inorganic Se (54–75%) mainly in form of selenate as suggested by LCF (Table 4) and the presence of a strong peak at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 668.1 eV in the XAS spectra of roots and leaves (Fig. 3). The shoulder at 12
668.1 eV in the XAS spectra of roots and leaves (Fig. 3). The shoulder at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 662 eV is fitted as organic Se (25–44%). The Se speciation in wheat from Field-1 and Indian mustard is listed in Table 4 for comparison. For more details on the speciation of these plants, please refer to Eiche et al.84
662 eV is fitted as organic Se (25–44%). The Se speciation in wheat from Field-1 and Indian mustard is listed in Table 4 for comparison. For more details on the speciation of these plants, please refer to Eiche et al.84
In the clover stem, inorganic Se is indicated to be dominant (81%) with a distinct peak at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 668.1 eV. LCF suggests selenate to be present in the stem with 64%. In the leaves, organic Se is slightly dominating according to the LCF with 58% and indicated by a shoulder at 12
668.1 eV. LCF suggests selenate to be present in the stem with 64%. In the leaves, organic Se is slightly dominating according to the LCF with 58% and indicated by a shoulder at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 662 eV (Fig. 3). Selenite seems to be of minor importance with 15–17% (Table 4).
662 eV (Fig. 3). Selenite seems to be of minor importance with 15–17% (Table 4).
Discussion
Processes influencing Se distribution and speciation in soil
All top and root zone soils can be classified as seleniferous at our study sites based on the threshold value of 0.5 mg Se per kg (ref. 71) with Se concentrations ranging from 2.0 to 13.0 mg kg−1 (Fig. 1 and Table S2†). The Setot concentrations in deeper soil horizons (>15 cm) are clearly lower (0.6 ± 0.1 mg kg−1, Table S2†). Interestingly, both the residual Se concentration in the enriched upper layers (Table 2) and the Setot in the deeper layers are in a similar range (Table S2†) indicating that this is the natural Se content in the parent soil material of the area.Our results strongly suggest that irrigation is the decisive process that controls the excess Se input into the soils which is in agreement with earlier studies.21,22,63 Accordingly, the three times higher average Setot content in the topsoil of Field-1 compared to Field-3 (Table 1) can partially be explained by the higher water demand for the rice-wheat rotation at Field-1. Calcium and especially Sr, which have high concentrations in the irrigation water (Table S9†),57 are also enriched in topsoils and with a similar depth distribution to Se (Fig. 1). This further supports a considerable influence of irrigation on the excess soil geochemistry. Apart from irrigation, the Setot content of Field-1 is probably influenced by its location in a small dip next to an irrigation channel. Here, Se rich water both from the field and leaking in from the irrigation channel itself can accumulate, which locally enhances the natural soil Se content. Leaching of Se deeper than the plough layer is not indicated to be of importance at our study sites.
Not only the Setot content but also the indicated Se speciation in the soils varies both in time and in space. We can observe a significant shift in Se speciation from irrigation water, where selenate is reported to be the sole species,22 towards less mobile species in all soils that were investigated (Table 2). The relatively low proportion of easily available Se (5–15%), which can mainly be assigned to selenate in the soils,65 indicates that immediate and preferential plant uptake of irrigation induced selenate and its transformation into less mobile species within the soil considerably changes the primary Se speciation over time. Similar has been observed by Schilling et al.22 in Punjab or other studies that were carried out in the context of biofortification.69,72 With regard to Se species transformation, biotic and abiotic reduction of selenate are of importance.22,37 In a first step, selenate is reduced to less mobile selenite,35,36 a fraction that accounts for only 4–8% (Fraction 2) in our soils. Still, the presence of selenite has also been proven by single particle XAS-analysis (Fig. 2). The low proportion of selenite could partly be explained by a diminished sorption capacity of alkaline soils for oxyanions (Table 1). More importantly, selenite can be further reduced to elemental Se, which is indicated to be a major species at most of our sites with 23–58% (Fraction 3, Table 2). Bajaj et al.42 showed that certain microbes (Duganella sp., Agrobacterium sp.) isolated from the same soils in Punjab are capable of reducing selenite to nano-particulate elemental Se. In addition, inorganic Se0 formation from dissolved selenite and selenate can be observed during the transformation of Fe-oxides,73 which is a common process in soils. Consequently, reduction to elemental Se can be considered as major immobilization and thus enrichment process of Se at our sites. Selenium can also be incorporated into Fe oxyhydroxides during oxidizing/reducing cycles as indicated by the relatively high proportion of Fraction 5 (14–43%; Table 2). Additionally, we assume that selenate that has been co-precipitated with calcite after irrigation is of importance in this fraction; a process which has already been proposed by Eiche.74 The fact that Se concentrations in the top 2 cm are depleted in comparison to the underlying 5–10 cm indicates that biomethylation and volatilization of Se might be another microbial transformation processes that actually removes Se from the soils. Selenium associated with organic matter is also of importance as indicated by a share of 9–18% (Fraction 4). This association is further supported by the strong correlation of Setot and Corg in all three depth profiles (rP > 0.98, n = 7, Fig. 1). Organic matter can have a considerable influence on the mobility of Se either via direct complexation of Se,75 the formation of organo–mineral associations that protect adsorbed Se or by fuelling the creation of localized anoxic zones.76 From our study, we cannot distinguish between organic Se species that result from the decomposition of Se enriched plant material (Table 4) or Se that is interacting with organic molecules.
The share of individual Se species is strongly influenced by the agricultural practice. We assume that the high proportion of easily available Se (22%) in the root zone soil of the wheat showing pink chlorosis can be explained by a diminished removal of bioavailable Se from the respective soil due to a reduced number of crop plants growing on the small ridge where the plant was taken. Consequently, the balance between selenate input (dissolved Se concentration & amount of irrigation water) and consumption (number of Se consumers per area unit) in addition to the rate of transformation determines the actual selenate content in the soil at a certain location.
The transformation processes are strongly influenced by the dominant irrigation regime. At least partly reducing conditions, which can develop during stagnant periods of rice cultivation, explain the much higher proportion of elemental Se (58%) at Field-1 compared to Field-3 (23%) (Table 2). The importance of elemental Se at Field-1 has already been proposed by Eiche74 and is further confirmed by the bulk and single particle XAS measurements that clearly indicate elemental Se as major Se species (Fig. 2). Fields under maize-wheat rotation, however, are dominated by Se that is oxidically/carbonate bound (43%). The relatively high proportion of elemental Se in all soils independent of its land use could be due to the fact that localized reducing microzones develop in soil aggregates independent of the bulk redox state when sufficient organic matter is available.77 An overestimation of elemental Se at the expense of organically associated Se65 is also conceivable. This could partly explain our much lower share of organically bound Se compared to Schilling et al.22 who found up to 80% of this fraction in topsoils of other sites in Punjab. However, also sampling at different plant growth states could explain the differences in Se speciation between our study and Schilling et al.22
Selenium content in plants and its relation to soil
The total Se content in different plant parts is alarmingly high with up to 931 mg kg−1 in known accumulators and up to 738 mg kg−1 in non-accumulators (Table 3). Especially the latter is remarkable as non-accumulators are reported to rarely exceed Se contents of 50 mg kg−1.49,50 Even in seleniferous areas, Se concentrations in these plants are typically below 25 mg Se per kg.51 In our study however, Se in clover, wheat (except Field-3) and sugarcane are 1.2 to 30 times higher compared to this value (Table 3). Still, the concentrations are mainly in a range that has been reported by other studies from the area.22,55,57,71,78 There is a clear positive relation between Setot in soils (rP = 0.89, n = 6) or the IR (rP = 0.90, n = 6), as known indicative parameter for bioavailable Se in soils,68 and the Semean in the plants, when Indian mustard and wheat showing pink chlorosis are not taken into account. Thus, both the bioavailable and Setot content in soils could be first indicators to predict possible Se enrichment in plants at our sites. The actual extent of plant Se enrichment, however, is clearly depending on the plant species itself due to genetic differences.49 In our study, this becomes apparent when comparing wheat from Field-2 and sugarcane, which differ in their Semean in plant material by nearly 100% (34, 63 mg per kg dw) despite similar Setot in the soils (5.2, 4.7 mg kg−1) (Tables 1 and 3).The plants can be separated into two groups based on the Se distribution within the plant. While wheat showing white and pink chlorosis as well as Indian mustard are clearly Se enriched in the upper plant parts by a factor 2 to 5 compared to the roots, the Se content in the other plants is more evenly distributed (Table 3). The considerable Se concentrations in all plant roots point towards a substantial uptake of organic Se or selenite.79 Especially the latter is known to be retained in roots via quick transformation into organic species.80–82 Our assumption is supported by the fact that organic Se is indicated to be the dominant form of Se in all roots except the wheat showing pink chlorosis (Table 4). The strong enrichment of Se in leaves of white and pink coloured wheat and Indian mustard (leave/root ratio > 1.4) suggests a substantial uptake and inner-plant transport of Se in form of selenate79,82–84 despite the relatively low indicated importance by sequential extraction (Fraction 1, Table 2). The lowest IR of all soils at the two sites, however, (Table 1) supports a relatively high Se bioavailability.68 It is important to remember in this context that all speciation related methods can only capture the current situation but cannot provide information about Se species that have already been taken up. We assume that intensive irrigation like at Field-1 leads to a regular and considerable input of fresh and thus highly bioavailable selenate that is mainly not retained in the soil but quickly taken up and transferred to upper plant parts. This assumption is further confirmed by the high proportion of Se as selenate that can be found in the leaves of these three plants (both wheat plants, Indian mustard) with Se dominance in upper plant parts (Table 4). Due to a different form of land use, all other sites are less (Field-2, 3, sugarcane) or not even intentionally irrigated (clover, garlic) so that the original pool of mobile selenate and its regular replenishment is much smaller. Consequently, uptake of Se in form of selenite or organic species is probably higher or equal to selenate at these locations.
Selenium speciation and toxicity in plants
So far, it is not possible to define a reliable threshold value above which plants develop signs of toxicity such as chlorosis, withering or stunted plant growth.38 This might in parts be explainable by the fact that only wheat plants show clear Se concentration related signs of chlorosis while many other plants form less clear symptoms like leaf tin burning or brown spots on the leaves.57In our study, wheat plants without visible signs of chlorosis have Se concentrations below 50 mg kg−1, which is at least four times less than in wheat that shows clear signs of toxicity. This is in accordance with other authors who reported toxicity symptoms for wheat with more than 100 mg Se per kg (ref. 57) and more than 325 mg Se per kg.85 Interestingly, wheat showing signs of white and pink chlorosis have similar Se concentrations in their roots and stems (Table 3). In the pinkish wheat, however, both the Se concentration (twice as high) and the proportion of inorganic Se (30% more) is clearly higher than in the whitish leaves (Tables 3 and 4). This indicates that the type of chlorosis is largely controlled by the extent of inorganic Se uptake probably as selenate which is the more bioavailable form and indicated to be present in these soils with a relatively high proportion (Tables 1 and 2). These findings also go in line with earlier studies, which indicate a reduced transformation to organic Se species with increasing selenate uptake.84,86 Instead, excess selenate that is transported to the leaves can be sequestered in vacuoles of cells.87 However, plants seem to face problems with the high selenate content despite its possible storage in vacuoles as indicated by the pink chlorosis.
Clover shows no visible signs of toxicity despite the high proportion of inorganic Se especially in the stem (81%) but also in the leaves (42%) (Table 4). Inorganic Se, which is taken up, seems to largely be transported through the stem to the leaves where about 50% are indicated to be transformed to organic Se species. Due to the much lower total Se content compared to the pinkish wheat (Table 3) the plant seems to be able to transform a much higher proportion of inorganic to organic Se species (Table 4). This could also be one reason for the lack of visible toxicity signs but clover is also reported to have a low tendency to develop Se related chlorosis.57 The comparable Se concentrations in all plant parts despite the dominance of mobile selenate in the stem could indicate that volatilization of Se is of importance in the leaves. This would also partly explain the high proportion of organic Se in the leaves.
Despite similar mean Se concentrations to clover, a much smaller proportion of inorganic Se, and nearly no selenate, can be identified in the sugarcane (Table 4). This fits to the results of the sequential extraction where sugarcane has the lowest proportion of easily available Se (Table 2) and the highest IR (Table 1). Therefore, either no considerable amount of selenate has been taken up or it already has been largely transferred into organic Se species. The high proportion of organic Se might partly be related to biomethylation of Se as Schilling et al.22 found indications that this is a considerable metabolic process in sugarcane from the area. Dhillon & Dhillon88 showed that the volatilization rate of sugarcane (6.84 to 31.5 mg per day per ha) is 5–6 times higher compared to other investigated crops like wheat, rice or mustard.
Implications for biofortification measures
Agronomic biofortification measures are a possible choice to raise dietary Se levels in Se deficient areas. Adding selenate to soil combined with fertilizer is one option that has been carried out in the past (e.g.ref. 89 and 90). Even though this method has proven to be highly successful in raising dietary Se concentrations, only 5–20% of the added Se has finally been detected in the plants (e.g.ref. 72, 91 and 92). Due to the relatively low annual Se addition (10–20 g ha−1) the time frame since the first application of selenate in biofortification measures (20–30 years) has been too short to allow reliable conclusions about the environmental fate of the remaining 80–95% of the added Se.72,92,93 In our study, the interval between first selenate input and detectable changes is considerably shortened due to the large quantity of irrigation induced Se (2.6 to 5.2 kg ha−1) and can thus be taken as helpful case study to resolve questions about the biofortification influenced Se cycling.Our study confirms that the input of selenate into the soil-plant system in Se deficient areas is probably the most effective and immediate way to assure a sufficiently high dietary Se level54,89 especially if food derives from above ground plant parts; but probably also the one with highest risks if improperly applied. As many plants are prone to easily take up large amounts of selenate,79 already a small shift in the balance of Se uptake vs. removal towards selenate uptake (e.g. low number of Se consumers on a field) or a slight change in mobility influencing parameters (e.g. pH-value, redox-conditions, microbial communities, competing ions) can cause potentially toxic Se levels in plants and the food products made from it. Especially wheat, which plays an important role in the production of Se-rich food,90 is able to significantly increase dietary Se concentrations if selenate is supplied. Of further concern is the high probability that enhanced selenate availability will lead to (1) a change in Se speciation from less toxic organic to more toxic inorganic Se species in food and forage,2,16 (2) poor harvests due to diminished plant health and (3) unhealthy livestock.62 Consequently, selenate based biofortification has to be carried out with care to avoid selenosis, economic losses and a waste of Se resources if large amounts of added Se are volatilized.
In accordance with other studies (e.g.ref. 72 and 89), we can demonstrate that a major part of selenate that is introduced into the soil via biofortification will be transferred into other, less mobile Se species thus creating a long-term (selenite, organic Se species, Se in carbonates) and non-available (elemental Se) Se pool. Darcheville et al.41 showed that microbial assisted processes are of special importance in this context. The individual share of the different Se species will depend on the form of land use, the small scale field morphology and the soil mineralogy and biogeochemistry. Selenate that is not immediately taken up by the plants will be retained in the soil through species transformation eventually causing a severe Se enrichment. Additionally, inorganic Se that is transformed to organic species within the plants will also return to the soil if not removed by harvest. The strong enrichment of organic Se in all roots at our study sites, however, clearly indicates that considerable transfer probably of selenite and/or organic Se into plants can take place despite the reduction in mobility.
Conclusions
Our study shows that soils play an important role in controlling the Se cycling in the critical zone as they can both act as sink and source of Se. A similar background Se concentration in all soils clearly indicates that the high soil Se concentrations in the uppermost part of the soils were not naturally present but result from secondary processes. In this context, irrigation water, where selenate is the sole species, is the primary source of excess Se and thus strongly influences the total Se concentration not only in the soil but also in the plants to which Se is largely transferred. Secondary processes that control the Se enrichment and speciation in the soils are: selective removal of Se species by plant uptake, accumulation and redistribution through microbial Se transformation and degradation of Se enriched organic matter. Agriculture, especially the form of land use, strongly influences all processes and is thus, a major factor in the local Se cycling.Selenium contents in plants within the study area are alarmingly high and could pose a severe threat if used as forage for livestock. A reliable prediction of plant Se concentrations is still not possible. Our results demonstrate that even knowledge about Se speciation in soils is not sufficient to explain or even predict the Se transfer into plants. Extractions, like all other speciation-characterizing techniques, can only provide a snapshot of the situation in the soil at the time of sampling but cannot provide information about the change in speciation in the course of the year. Especially external input of highly mobile selenate, for example through irrigation or biofortification measures, is not necessarily captured in the soil speciation if quickly taken up but will have severe impact on plant Se content and plant health.
Our results demonstrate that biofortification measures using selenate should be carried out with care. Quick success in Se enhancement in food products has to be balanced with danger of chlorosis, less favourable Se speciation in food and general and long-term increase of Se in the plant-soil system. Loss of Se to the atmosphere due to volatilization makes the whole measure less economic and thus is a waste of resources if not carried out with care.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We want to thank the International Bureau of the BMBF for financially supporting the sampling campaign in Punjab and, thus, enable the cooperation with our Indian colleagues. Furthermore, we would like to thank Dr Mini Bajaj from the former Institute of Biology for Engineers and Biotechnology of Wastewater (KIT), for her important support to start up the cooperation and during the research trip to Ludhiana. Many thanks also to Prof. Dr Upkar Sadana for his invitation and his help during our stay. The work would not have been possible without the support of many colleagues from the Department of Soil from the PAU, thanks for that. We also want to acknowledge the support of all technicians in the Institute of Applied Geosciences at KIT who helped during the analytical work namely Claudia Mössner, Beate Oetzel, Cornelia Haug and Kristian Nikoloski.References
- H. Hartikainen, Biogeochemistry of selenium and its impact on food chain quality and human health, J. Trace Elem. Med. Biol., 2005, 18, 309–318 CrossRef CAS PubMed.
- D. G. Barceloux and D. Barceloux, Selenium, J. Toxicol., Clin. Toxicol., 1999, 37, 145–172 CrossRef CAS PubMed.
- C. Ip, C. Hayes, R. M. Budnick and H. E. Ganther, Chemical form of selenium, critical metabolites, and cancer prevention, Cancer Res., 1991, 51, 595–600 CAS.
- M. Vinceti, T. Filippini, S. Cilloni, A. Bargellini, A. V. Vergoni, A. Tsatsakis and M. Ferrante, Health risk assessment of environmental selenium: Emerging evidence and challenges (Review), Mol. Med. Rep., 2017, 15, 3323–3335 CrossRef CAS PubMed.
- M. P. Rayman, The importance of selenium to human health, Lancet, 2000, 356, 233–241 CrossRef CAS.
- F. Fordyce, Selenium Geochemistry and Health, Ambio, 2007, 36, 94–97 CrossRef CAS PubMed.
- M. Navarro-Alarcon and C. Cabrera-Vique, Selenium in food and the human body: a review, Sci. Total Environ., 2008, 400, 115e141 CrossRef PubMed.
- World Health Organisation (WHO), Trace elements in human nutrition and health, WHO Press, Geneva, 1996 Search PubMed.
- K. T. Suzuki, Metabolomics of selenium: Se metabolites based on speciation studies, J. Health Sci., 2005, 51, 107e114 Search PubMed.
- O. A. Levander and R. F. Burk, Update of human dietary standards for selenium, in Selenium: Its molecular biology and role in human health, ed. D. L. Hatfield, M. J. Berry and V. N. Gladyshev, Springer, New York, 2nd edn, 2006, pp. 399–410 Search PubMed.
- M. P. Rayman, Food-chain selenium and human health: emphasis on intake, Br. J. Nutr., 2008, 100, 254–268 CrossRef CAS PubMed.
- M. P. Rayman, Selenium and human health, Lancet, 2012, 379, 1256–1268 CrossRef CAS.
- National Research Council (NRC), Mineral Tolerance of Domestic animals, Commission on Natural Resources, National Academy of Sciences, Washington DC, 1980 Search PubMed.
- L. Wu, P. J. V. Mantgem and X. Guo, Effects of forage plant and field legume species on soil Se redistribution, leaching and bioextraction in soils contaminated by agricultural drain water sediment, Arch. Environ. Contam. Toxicol., 1996, 31, 329–338 CrossRef CAS.
- L. L. Miller and A. Hontela, Species-specific sensitivity to selenium-induced impairment of cortisol secretion in adrenocortical cells of rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis), Toxicol. Appl. Pharmacol., 2011, 253, 137–144 CrossRef CAS PubMed.
- J. E. Spallholz, On the nature of selenium toxicity and carcinostatic activity, Free Radical Biol. Med., 1994, 17, 45–64 CrossRef CAS PubMed.
- M. P. Rayman, H. G. Infante and M. Sargent, Food-chain selenium and human health: spotlight on speciation, Br. J. Nutr., 2008, 100, 238–253 CrossRef CAS PubMed.
- J. F. Combs, Selenium in global food system, Br. J. Nutr., 2001, 85, 517–547 CrossRef PubMed.
- E. Dumont, F. Vanhaecke and R. Cornelis, Selenium speciation from food source to metabolites: a critical review, Anal. Bioanal. Chem., 2006, 385, 1304–1323 CrossRef CAS PubMed.
- C. Reilly, Selenium in Food and Health, Springer, New York, 2nd edn, 2006, p. 206 Search PubMed.
- M. Bajaj, E. Eiche, T. Neumann, J. Winter and C. Gallert, Hazardous concentrations of selenium in soil and groundwater in North-West India, J. Hazard. Mater., 2011, 189(3), 640–646 CrossRef CAS PubMed.
- K. Schilling, T. M. Johnson, K. S. Dhillon and P. R. D. Mason, Fate of Selenium in Soils at a Seleniferous Site Recorded by High Precision Se Isotope Measurements, Environ. Sci. Technol., 2015, 49, 9690–9698 CrossRef CAS PubMed.
- U.S. Environmental Protection Agency (USEPA), Water quality Criteria 1972, EPA.R3.73.033, March 1973 Search PubMed.
- Ontario Ministry of the Environment (OMOE), Water management, goals, policies, objectives and implementation procedures of the Ministry of the Environment, Toronto, Ont, 1984, Revised, p. 70 Search PubMed.
- N. K. Nagpal and K. Howell, Water Quality Guidelines for Selenium – Technical Appendix, Prepared for the Ministry of Water, Lands and Air Protection (MWLAP), Victoria, British Columbia, 2001 Search PubMed.
- J. A. Plant, D. G. Kinniburgh, P. L. Smedley, F. M. Fordyce and B. A. Klinck, Arsenic and Selenium, in Treatise on Geochemistry, Environmental Geochemistry, ed. H. D. Holland and K. K. Turekian, Elsevier, Amsterdam/Heidelberg, 2014, vol. 11, pp. 13–57 Search PubMed.
- D. J. Swaine, The trace-element content of soils, Harpenden, England, Common. Bur. Soil Sci. Tech. Commun., 1955, 48, 91.
- D. C. Adriano, Trace Elements in the Terrestrial Environment, Springer, 2nd edn, Berlin, 2001 Search PubMed.
- S. K. Dhillon, B. K. Hundal and D. S. Dhillon, Bioavailability of selenium to forage crops in a sandy loam soil amended with Se-rich plant materials, Chemosphere, 2007, 66, 1734–1743 CrossRef CAS PubMed.
- M. Lenz and P. N. L. Lens, The essential toxin: The changing perception of selenium in environmental sciences, Sci. Total Environ., 2009, 407(12), 3620–3633 CrossRef CAS PubMed.
- T. Blazina, Y. Sun, A. Voegelin, M. Lenz, M. Berg and L. H. E. Winkel, Terrestrial selenium distribution in China is potentially linked to monsoonal climate, Nat. Commun., 2014, 5, 4717 CrossRef CAS PubMed.
- Z. J. Wang and Y. X. Gao, Biogeochemical cycling of selenium in Chinese environments, Appl. Geochem., 2001, 16, 1345–1351 CrossRef CAS.
- L. H. E. Winkel, B. Vriens, G. D. Jones, L. S. Schneider, E. Pilon-Smits and G. S. Bañuelos, Selenium Cycling Across Soil-Plant-Atmosphere Interfaces: A Critical Review, Nutrients, 2015, 7, 4199–4239 CrossRef CAS PubMed.
- A. Haug, R. D. Graham, O. A. Christophersen and G. H. Lyons, How to use the world's scarce selenium resources efficiently to increase the selenium concentration in food, Microb. Ecol. Health Dis., 2007, 19, 209–228 CrossRef CAS PubMed.
- A. Fernández-Martínez and L. Charlet, Selenium environmental cycling and bioavailability: a structural chemist point of view, Rev. Environ. Sci. Biotechnol., 2009, 8, 81–110 CrossRef.
- J. A. Ippolito, K. G. Scheckel and K. A. Barbarick, Selenium adsorption to aluminium-based water treatment residuals, J. Colloid Interface Sci., 2009, 338, 48–55 CrossRef CAS PubMed.
- J. F. Stolz and R. S. Oremland, Bacterial respiration of arsenic and selenium, FEMS Microbiol. Rev., 1999, 23, 615–627 CrossRef CAS PubMed.
- Natasha, M. Shahid, N. K. Niazi, S. Khalid, B. Murtaza, I. Bibi and M. I. Rahid, A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health, Environ. Pollut., 2018, 234, 915–934 CrossRef CAS PubMed.
- R. S. Oremland, J. T. Hollibaugh, A. S. Maest, T. S. Presser, L. G. Miller and C. W. Culbertson, Selenate reduction to elemental selenium by anaerobic-bacteria in sediments and culture-biogeochemical significance of a novel, sulfate-independent respiration, Appl. Environ. Microbiol., 1989, 55, 2333–2343 CAS.
- C. S. Haudin, P. Renault, E. Leclerc-Cessac and S. Staunton, Effect of selenite additions on microbial activity and dynamics in three soils incubated under aerobic conditions, Soil Biol. Biochem., 2007, 39, 2670–2674 CrossRef CAS.
- O. Darcheville, L. Février, F. Z. Haichar, O. Berge, A. Martin-Garin and P. Renault, Aqueous, solid and gaseous partitioning of selenium in an oxic sandy soil under different microbiological states, J. Environ. Radioact., 2008, 99, 981–992 CrossRef CAS PubMed.
- M. Bajaj, S. Schmidt and J. Winter, Formation of Se (0) Nanoparticles by Duganella sp. and Agrobacterium sp. Isolated from Se-laden soil of North-East Punjab, India, Microb. Cell Fact., 2012, 11, 64 CrossRef CAS PubMed.
- W. T. Frankenberger and U. Karlson, Soil-management factors affecting volatilization of selenium from dewatered sediments, Geomicrobiol. J., 1994, 12, 265–278 CrossRef CAS.
- W. T. Frankenberger and M. Arshad, Bioremediation of selenium-contaminated sediments and water, BioFactors, 2001, 14, 241–254 CrossRef CAS.
- M. M. Abrams, C. Shennan, R. J. Zasoski and R. G. Burau, Selenomethionine uptake by w heat seedlings, Agron. J., 1990, 82, 1127–1130 CrossRef CAS.
- J. Kikkert and E. Berkelaar, Plant uptake and translocation of inorganic and organic forms of selenium, Arch. Environ. Contam. Toxicol., 2013, 65, 458–465 CrossRef CAS PubMed.
- Y. Ogra, Y. Ogihara and Y. Anan, Comparison of the metabolism of inorganic and organic selenium species between two selenium accumulator plants, garlic and Indian mustard, Metallomics, 2017, 9, 61–68 RSC.
- G. Alfthan, M. Eurola, P. Ekholm, E.-R. Venäläinen, T. Root, K. Korkalainen, H. Hartikainen, P. Salminen, V. Hietaniemi, P. Aspila and A. Aro, Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: from deficiency to optimal selenium status of the population, J. Trace Elem. Med. Biol., 2015, 31, 142–147 CrossRef CAS PubMed.
- I. Rosenfeld and O. A. Beath, Selenium: Geobotany, Biochemistry, Toxicity and Nutrition, Academic Press, New York, 1964 Search PubMed.
- H. F. Mayland, L. F. James, K. E. Panter and J. L. Sonderegger, Selenium in seleniferous environments, Soil Sci. Soc. Am. J., 1989, 23, 15–50 CAS.
- N. Terry, A. M. Zayed, M. P. De Souza and A. S. Tarun, Selenium in higher plants, Annu. Rev. Plant Physiol. Plant Mol. Biol., 2000, 51, 401–432 CrossRef CAS PubMed.
- T. A. Brown and A. Shrift, Selenium: toxicity and tolerance in higher plants, Biol. Rev., 1982, 57, 59–84 CrossRef CAS.
- H. Marschner, Mineral Nutrition of Higher Plants, Academic Press, London, 1995, pp. 430–433 Search PubMed.
- F. M. Fordyce, Selenium Deficiency and Toxicity in the Environment, Essentials of Medical Geology, Springer, 2013, pp. 375–416 Search PubMed.
- K. S. Dhillon and S. K. Dhillon, Selenium toxicity in soils, plants and animals in some parts of Punjab, India, Int. J. Environ. Stud., 1991, 37, 15–24 CrossRef CAS.
- K. S. Dhillon, S. S. Bawa and S. K. Dhillon, Selenium toxicity in some plants and soils of Punjab, J. Indian Soc. Soil Sci., 1992, 40, 132–136 CAS.
- K. S. Dhillon and S. K. Dhillon, Characterization and Management of Seleniferous Soils of Punjab, Research Bulletin No. 1/2009, Punjab University, Department of Soils. Ludhiana, India, 2009 Search PubMed.
- S. D. Atri and A. Tyagi, Climate Profile of India. Contribution to the Indian Network of Climate Change Assessment (NATIONAL COMMUNICATION-II), Ministry of Environment and Forests Government of India Ministry of Earth Sciences, India Meteorological Department Met Monograph No. Environment Meteorology-01/2010, New Delhi, 2010 Search PubMed.
- K. S. Dhillon and S. K. Dhillon, Quality of underground water and its contribution towards selenium enrichment of the soil-plant system for a seleniferous region of northwest India, J. Hydrol., 2003, 272, 120–130 CrossRef CAS.
- S. K. Dhillon and K. S. Dhillon, Pools of Selenium in some Indian soils at field capacity and submerged moisture regimes, Aust. J. Soil Res., 2004, 42, 247–257 CrossRef CAS.
- K. S. Dhillon and S. K. Dhillon, Distribution of seleniferous soils in north-west India and associated toxicity problems in the soil-plant-animal-human continuum, Land Contamination and Reclamation, 1997, vol. 5, pp. 313–322 Search PubMed.
- C. K. Hira, K. Partal and K. S. Dhillon, Dietary selenium intake by men and women in high and low selenium areas of Punjab, Public Health Nutr., 2004, 7, 39–43 CrossRef.
- K. S. Dhillon and S. K. Dhillon, Development and mapping of seleniferous soils in northwestern India, Chemosphere, 2014, 99, 56–63 CrossRef CAS PubMed.
- Y. Zhang and J. Moore, Selenium fractionation and speciation in a wetland system, Environ. Sci. Technol., 1996, 30, 2613–2619 CrossRef CAS.
- M. T. Wright, D. R. Parker and C. Amrhein, Critical evaluation of the ability of sequential extraction procedures to quantify discrete forms of selenium in sediments and soils, Environ. Sci. Technol., 2003, 4709–4716 CrossRef CAS.
- J. R. Bacon and C. M. Davidson, Is there a future for sequential extraction?, Analyst, 2008, 24–46 Search PubMed.
- F. X. Han, A. Banin, W. L. Kingery, G. B. Triplett, L. X. Zhou, S. J. Zheng and W. X. Ding, New approach to studies of redistribution of heavy metals in soils, Adv. Environ. Res., 2003, 8, 113–120 CrossRef CAS.
- Q. Peng, L. Guo, F. Ali, J. Li, S. Qin, P. Feng and D. Liang, Influence of Pak Choi plant cultivation on Se distribution, speciation and bioavailability in soil, Plant Soil, 2016, 403, 331–342 CrossRef CAS.
- F. Ali, Q. Peng, D. Wang, Z. Cui, J. Huang, D. Fu and D. Liang, Effects of selenite and selenate application on distribution and transformation of selenium fractions in soil and its bioavailability for wheat (Triticum aestivum L.), Environ. Sci. Pollut. Res., 2017, 24, 8315–8325 CrossRef CAS PubMed.
- B. Ravel and M. Newville, ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT, J. Synchrotron Radiat., 2005, 12, 537–541 CrossRef CAS.
- K. S. Dhillon and S. K. Dhillon, Accumulation of selenium in sugarcane (Sacchharum officinarum Linn.) in seleniferous areas of Punjab, India, Environ. Geochem. Health, 1991, 13, 165–170 CrossRef CAS PubMed.
- A. W. Mathers, S. D. Young, S. P. McGrath, F. J. Zhao, N. M. J. Crout and E. H. Bailey, Determining the fate of selenium in wheat biofortification: an isotopically labelled field trial study, Plant Soil, 2017, 420, 61–77 CrossRef CAS.
- N. Börsig, A. C. Scheinost, S. Shaw, D. Schild and T. Neumann, Uptake mechanisms of selenium oxyanions during the ferrihydrite-hematite recrystallization, Geochim. Cosmochim. Acta, 2016, 206, 236–253 CrossRef.
- E. Eiche, Microscale distribution and elemental associations of Se in seleniferous soils in Punjab, India, Environ. Sci. Pollut. Res., 2015, 22, 5425–5436 CrossRef CAS PubMed.
- J. P. Gustafsson and L. Johnson, Selenium retention in the organic matter of Swedish forest soils, J. Soil Sci., 1992, 43, 461–472 CrossRef CAS.
- J. Tolu, Y. Thiry, M. Bueno, C. Jolivet, M. Potin-Cautier and I. Le Hécho, Distribution and speciation of ambient selenium in contrasted soils, from mineral to organic rich, Sci. Total Environ., 2014, 479–480, 93–101 CrossRef CAS PubMed.
- M. Kausch, P. Ng, J. Ha and C. Pallud, Soil-aggregate-scale heterogeneity in microbial selenium reduction, Vadose Zone J., 2012, 11(2), vzj2011.0101 Search PubMed.
- N. Sharma, R. Prakash, A. Srivastava, U. S. Sadana, R. Acharya, N. T. Prakash and A. V. R. Reddy, Profile of selenium in soil and crops in seleniferous area of Punjab, India by neutron activation analysis, J. Radioanal. Nucl. Chem., 2009, 281, 59–62 CrossRef CAS.
- A. K. Nothstein, E. Eiche, M. Riemann, P. Nick, L. H. E. Winkel, J. Göttlicher, R. Steininger, R. Brendel, M. von Brasch, K. Konrad and T. Neumann, Tracking Se Assimilation and Speciation through the Rice Plant – Nutrient Competition, Toxicity and Distribution, PLoS One, 2016, 11, e0152081 CrossRef PubMed.
- M. P. De Souza, E. A. H. Pilon-Smits, C. M. Lytle, S. Hwang, J. Tai, T. S. U. Honma, L. Yeh and N. Terry, Rate-limiting steps in selenium assimilation and volatilization by Indian mustard, Plant Physiol., 1998, 117, 1487–1494 CrossRef CAS PubMed.
- H.-F. Li, S. P. McGrath and F.-J. Zhao, Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite, New Phytol., 2008, 178, 92–102 CrossRef CAS PubMed.
- A. Zayed, C. M. Lytle and N. Terry, Accumulation and volatilization of different chemical species of selenium by plants, Planta, 1998, 206, 284–292 CrossRef CAS.
- M. Arvy, Selenate and selenite uptake and translocation in bean plants (Phaseolus vulgaris), J. Exp. Bot., 1993, 44, 1083–1087 CrossRef CAS.
- E. Eiche, F. Bardelli, A. Nothstein, L. Charlet, J. Göttlicher, R. Steininger, K. S. Dhillon and U. S. Sadana, Selenium distribution and speciation in plant parts of wheat (Triticum aestivum) and Indian mustard (Brassica juncea) from a seleniferous area of Punjab, India, Sci. Total Environ., 2015, 505, 952–961 CrossRef CAS PubMed.
- G. H. Lyons, J. C. R. Stangoulis and R. D. Graham, Tolerance of wheat (Triticum aestivum L.) to high soil and solution selenium levels, Plant Soil, 2005, 270, 179–188 CrossRef CAS.
- P. D. Whanger, Selenocompounds in plants and animals and their biological significance, J. Am. Coll. Nutr., 2002, 21, 223–232 CrossRef CAS PubMed.
- D. Mazej, J. Osvald and V. Stibilj, Selenium species in leaves of chicory, dandelion, lamb's lettuce and parsley, Food Chem., 2008, 107, 75–83 CrossRef CAS.
- S. K. Dhillon and K. S. Dhillon, Phytoremediation of selenium-contaminated soils: the efficiency of different cropping systems, Soil Use Manage., 2009, 25, 441–453 CrossRef.
- M. R. Broadley, P. J. White, R. J. Bryson, M. C. Meacham, H. C. Bowen, S. E. Johnson, M. J. Hawkesford, S. T. McGrath, F.-J. Zhao, N. Breward, M. Harriman and M. Tucker, Biofortification of UK food crops with selenium, Proc. Nutr. Soc., 2006, 65, 169–181 CrossRef CAS.
- M. Poblaciones, S. Rodrigo, O. Santamaría, Y. Chen and S. McGrath, Agronomic selenium biofortification in Triticum durum under Mediterranean conditions: from grain to cooked pasta, Food Chem., 2014, 146, 378e384 CrossRef.
- T. Yläranta, Raising the selenium content of spring wheat and barley using selenite and selenate, Ann. Agric. Fenn., 1984, 23, 75–84 Search PubMed.
- R. Keskinen, M. Räty and M. Yli-Halla, Selenium fractions in selenate-fertilized field soils of Finland, Nutr. Cycling Agroecosyst., 2011, 91, 17–29 CrossRef CAS.
- G. Alfthan, P. Aspila, P. Ekholm, M. Eurola, H. Hartikainen and H. Hero, et al., Nationwide supplementation of sodium selenate to commercial fertilizers. History and 25-year results from the Finnish selenium monitoring program, in Combating micronutrient deficiencies: food-based approaches, ed. B. Thompson and L. Amoroso, FAO/CAB International, Rome, 2010 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9em00009g |
| This journal is © The Royal Society of Chemistry 2019 |