A pilot study of metabolites of organophosphorus flame retardants in paired maternal urine and amniotic fluid samples: potential exposure risks of tributyl phosphate to pregnant women†
Xue-yuan
Bai‡
 ab,
Shao-you
Lu‡
ab,
Shao-you
Lu‡
 c,
Lei
Xie
c,
Lei
Xie
 a,
Bo
Zhang
a,
Bo
Zhang
 a,
Shi-ming
Song
a,
Shi-ming
Song
 a,
Yuan
He
a,
Yuan
He
 a,
Ji-ping
Ouyang
a,
Ji-ping
Ouyang
 a and
Tao
Zhang
a and
Tao
Zhang
 *ab
*ab
aSchool of Environmental Science and Engineering, Sun Yat-Sen University, 135 Xingang West Street, Guangzhou 510275, China. E-mail: zhangt47@mail.sysu.edu.cn; Tel: +86-22-84113454
bGuangdong Provincial Key Laboratory of Environmental Pollution Control and Remediation Technology (Sun Yat-Sen University), Guangzhou 510275, China
cShenzhen Center for Disease Control and Prevention, Shenzhen 518055, PR China
First published on 5th November 2018
Abstract
Organophosphorus flame retardants (OPs) are of wide concern due to their presence in human urine and their considerable endocrine disruption and neuro-development toxicity. It has been confirmed that electronic waste (e-waste) dismantling activities have contributed to human exposure to OPs. However, assessments of OP exposure and the health risks for pregnant women and fetuses living in areas associated with e-waste dismantling have been impeded by a lack of data. In this study, six OP metabolites (mOPs) were measured in paired maternal urine and amniotic fluid samples collected from an e-waste dismantling area in Guangdong Province, China. All mOPs were detectable in maternal urine, whereas two were found in amniotic fluid. Dibutyl phosphate (DBP) was the predominant mOP in both maternal urine (geometric mean (GM): 2.9 ng ml−1) and amniotic fluid (1.3 ng ml−1); and diphenyl phosphate (DPHP) was the secondary one found (0.94 ng ml−1 in maternal urine, 0.12 ng ml−1 in amniotic fluid). The GM urinary concentrations of DBP and DPHP were two and seven times higher than those in amniotic fluid, respectively. The estimated daily intakes (EDIs) of triphenyl phosphate (TPHP) and tributyl phosphate (TnBP) by pregnant women were calculated from their daily urine excretion rate as fractions of OP metabolized to the corresponding metabolite (FUE). Our results showed high exposure levels to TPHP (median: 273 or 613 ng per kg bw per day) and TnBP (404 ng per kg bw per day) for pregnant women living in the e-waste associated area. Most importantly, 13% of mothers had EDITnBP levels that exceeded the reference dose (RfD: 2400 ng per kg bw per day), suggesting potential health risks from TnBP exposure for pregnant women living in areas associated with e-waste dismantling. This study, as a pilot study, presents the first measurements of mOPs in human amniotic fluid.
Environmental significanceThe aim of this study into the exposure of pregnant women living in an e-waste dismantling area to organophosphorus flame retardants (OPs) is to provide an innovative interpretation of OP exposure via a sensitive exposome study of pregnant women in a high-risk exposure area and to study mother–fetal transmission in relation to amniotic fluid. It should enable us to change our thinking about the ways that fetuses are also exposed via a mother–fetal transmission route to contaminants via amniotic fluid and, importantly, how this may adversely affect the growth, differentiation and development of an embryo. This study is a starting point for furthering the understanding as to how OP exposure due to e-waste dismantling impacts pregnant women and fetuses and its potential role in human early development. |
1. Introduction
Organophosphate esters (OPs) are generally used for two reasons: halogenated (Cl-OPs) [e.g., tris(1-chloro-2-propyl)phosphate (TCIPP)] and non-halogenated (NCl-OPs) [e.g., tris(2-butoxyethyl)phosphate (TBOEP), tri-n-butyl phosphate (TnBP), and triphenyl phosphate (TPHP)] OPs have been widely used as flame retardants and plasticizers for decades, respectively, in a variety of industrial and consumer products, including textiles, plastics, electronics, upholstered furniture, and baby products.1–3 Thus, OPs have been detected in various matrices, including indoor air, indoor dust, drinking water and foodstuffs, suggesting high human exposure.4–7The recycling of electrical and electronic waste (e-waste) in developing countries has attracted significant attention as an important source of many toxic chemicals.8 It has been reported that around 80% of the world's e-waste is exported to mid-and low-latitude developing countries/regions in Asia, and 90% of this flows into China.8 Villagers and migrant workers from an e-waste dismantling area in Guangdong province use primitive and environmentally unacceptable techniques, such as open burning to separate and recover metals from printed circuit boards, polyvinyl chloride-coated wires and cables.9 This unsafe e-waste recycling practice causes severe environmental pollution.9,10 Studies have shown that e-waste dismantling activities can contribute to flame retardant, such as OPs, release into the environment, causing residents to be exposed.3,8–10 However, only one study has reported urinary metabolites of OPs (mOPs) in the general population living in the e-waste dismantling area,3 and no studies have reported the exposure level to OPs of sensitive groups, such as pregnant women and fetuses, living in this area.
Animal toxicity studies and epidemiological investigations have shown the adverse effects of OPs on the development of fetuses.11,12 TCIPP and TPHP exposure significantly increased p53 mRNA levels in human embryo liver L02 cells and induced apoptosis depending on the induction and activation of p53.11 TPHP could increase thyroid hormone concentrations in the early life stages of zebrafish by disrupting the central regulation and hormone synthesis pathways.12 Prenatal exposure is considered to be the most sensitive period in human development, in which fetal cells are the subjects of division and differentiation.13 The placental barrier can prevent some transmission of some harmful chemicals from mothers to fetuses, however, contaminants such as polychlorinated biphenyls (PCBs),14 polybrominated diphenyl ethers (PBDEs)15 and polycyclic aromatic hydrocarbons (PAHs)16 are known to be able to cross the placenta and enter the umbilical cord. Perfluorooctane sulfonate (PFOS),17 mercury13 and organochlorines18 were detected in amniotic fluid, suggesting maternal–fetal transmission. Thus, fetuses have high susceptibility to the environment, and the composition of amniotic fluid (amniotic fluid, as the direct environment in which fetuses live before birth, surrounds and protects developing embryos and fetuses19), which is primarily fetal urine, varies over the course of gestation, reflecting the sequential maturation of fetal organs and accompanying shifts in the sites of fetal metabolism and filtration.19 Although fetal lungs and kidneys excrete fluid continuously into the amniotic fluid reservoir, fetuses are also continuously swallowing and “inhaling” amniotic fluid.19 Therefore, amniotic fluid is likely to be a useful biomarker for chemical exposure measurements regarding fetuses.19 However, to date, no studies have reported the levels of OPs in human amniotic fluid, and studies of mother–fetal exposure correlation are also very limited.13,18,19 Hence, reports detailing prenatal OP exposure and risk assessments of pregnant women and fetuses living in e-waste dismantling areas are necessary.
In the present study, we collected 15 paired maternal urine and amniotic fluid samples from pregnant women in an e-waste dismantling area of Guangdong, China and determined the concentrations of 6 mOPs in these samples to identify the transmission of mOPs (or OPs) from mothers to their fetuses. Also, we evaluated the daily OP intake and exposure risks of mothers living in the e-waste dismantling area.
2. Materials and methods
2.1 Chemicals and reagents
Six OPs and corresponding mOPs were purchased from Toronto Research Chemicals (all had >97% purity, Toronto, Canada), including bis(1,3-dichloro-2-propyl)phosphate (BDCIPP), bis(2-butoxyethyl)phosphate (BBOEP), dibutyl phosphate (DBP), di-o-cresyl phosphate (DoCP), di-p-cresyl phosphate (DpCP), and diphenyl phosphate (DPHP) (their parent compounds are shown in Table S1†) and the corresponding internal standards of d10-BDCIPP, d8-BBOEP, d18-DBP, d14-DoCP, d14-DpCP, and d10-DPHP. Methanol and ethyl acetate were obtained from Anpel, Shanghai, China (CNW Technologies) at high-performance liquid chromatography (HPLC)-grade. Deionized water used in the experiments was obtained from a Millipore system (Billerica, MA). β-Glucuronidase was purchased from Sigma-Aldrich (MO, USA).2.2 Study areas and sample collection
The e-waste dismantling area involved in this study is located in Qingyuan City, Guangdong Province, China. In Qingyuan city, the e-waste dismantling industry has a history of about 40 years. At the end of 2014, there were more than 2300 small-scale retail investors engaged in metal dismantling in Qingyuan city, and the number of employees reached more than 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Our sampling location has a huge copper recycling industry; it is necessary to peel off the outer skin of other materials on the metal surface, mainly via mechanical or semi-manual means. The irregular dismantling process results in the release of OPs into the environment, which leads to human exposure.3,20
000. Our sampling location has a huge copper recycling industry; it is necessary to peel off the outer skin of other materials on the metal surface, mainly via mechanical or semi-manual means. The irregular dismantling process results in the release of OPs into the environment, which leads to human exposure.3,20
All experiments were performed in accordance with the relevant laws and institutional guidelines of China and approved by the Ethics Committee of Sun Yat-sen University. The purpose of the experiments and the uses of the samples were fully explained, and informed consent was obtained from the healthy pregnant participants of this study. Participants voluntarily signed the experimental information agreement and submitted a questionnaire relating to physical information. Fifteen paired maternal urine and amniotic fluid samples (total: n = 30) were collected during the year 2016–2017 in an e-waste dismantling area of Qingyuan City. Maternal urine samples were collected during the day and amniotic fluid samples were collected at the same time according to standard clinical procedures. All collected samples were immediately stored and kept frozen at −20 °C until extraction and chemical analysis.
2.3 Sample preparation
Fluid samples were prepared for mOP analysis as follows, with a method from previous studies with minor adjustment.21 Briefly, 2 ml of urine or amniotic fluid sample was transferred to a glass tube and spiked with 10 ng of internal standard solution. 1.0 M ammonium acetate buffer with 82 units of β-glucuronidase was added and digestion was carried out in a water bath at 37 °C for 12 h. The mixture samples were extracted three times; each time, 3 ml of ethyl acetate was added, the sample was shaken for 60 min and centrifuged at 5000 rpm for 5 min, and then the supernatant liquor was recovered. After that, the ethyl acetate solvent was washed with 1 ml of Milli-Q water. Finally, a nitrogen blower was used to gently blow samples to near dryness; they were then reconstituted with 0.5 ml of methanol, vortexed and mixed, and then transferred into a glass sample bottle and stored at −20 °C, waiting to be analyzed.2.4 Instrumental analysis
The instrumental analysis method was the same as in our previous study.3 Concentrations of mOPs in urine and amniotic fluid were measured using a 20A HPLC system (Shimadzu, Japan) interfaced with a Q-Trap 5500 mass spectrometer (Applied Biosystems, Foster City, CA). Detailed information regarding the analytes with the corresponding internal standards is listed in Table S1.† Separation of mOPs was conducted with an XTerra-C18 column (5 μm, 4.6 × 250 mm, Waters). The column temperature was maintained at 40 °C. Water containing 10 mM ammonium acetate and methanol was used as the mobile phase. The flow rate was set at 0.6 ml min−1 and the gradient elution was set as follows: 0–5 min: 55% methanol; 5–18 min: 55–68% methanol; 18–20 min: 68–100% methanol; 20–25 min: 100% methanol; 25–27 min: 100–55% methanol; and 27–30 min: 55% methanol. 10 μL of the extract was injected for each sample. Electrospray ionization was operated in negative ion mode. Multiple reaction monitoring modes were used for the quantification of all mOPs, with a dwell time of 50 ms. The ion source temperature was 450 °C and the ionization voltage was 4500 V. Optimized MS/MS parameters, including precursor ion, product ion, collision energies, declustering potential, entrance potential and collision cell exit potential, for each analyte and the corresponding internal standards are also shown in Table S1.† DoCP and DpCP were the metabolites of tricresyl phosphate (TCP), which could not be chromatographically separated; therefore, the sum concentrations of both mOPs are referred to as O + P in further discussion.2.5 Quality assurance and quality control
Laboratory quality assurance methods included one procedural blank (i.e., Milli-Q water extraction) and one instrumental blank (i.e., methanol injection), which were prepared for every 10 samples, in order to simultaneously monitor contamination from reagents, glassware and instruments. No detectable levels of any analyzed mOP were found in the blanks. Matrix-spike recoveries of individual mOPs through the analytical procedure were determined by spiking (10 ng each) six mOPs into randomly selected urine samples; the recoveries of mOPs ranged from 75% ± 9% (DBP) to 113% ± 12% (BDCIPP). Calibration curves were prepared using standard solutions of the target analytes over a concentration range of 0.01–100 ng ml−1. The calibration curves of all individual mOPs had regression coefficients (r2) above 0.99. The stability of the detector response during instrumental analysis was checked by using moderate levels of mixed internal standard solution (10 ng ml−1), confirming a relative standard deviation (RSD) of less than 10%. The limits of quantification (LOQs), defined as ten times the signal-to-noise (S/N) ratio, ranged from 0.10 to 0.50 ng ml−1 among the analyzed mOPs: 0.50 (BDCIPP); 0.10 (BBOEP); 0.10 (DBP); 0.10 (O + P); and 0.10 (DPHP) ng ml−1.2.6 Statistical analysis
A value of LOQ/2 was used for concentrations below the LOQ (<LOQ) when calculating the geometric mean (GM), median and mean value.22 Data analysis was performed with SPSS Version 19.0. Urinary (or amniotic fluid) concentrations of mOPs were tested for normality using the Kolmogorov–Smirnov test. Log-transformations were used for correlation analyses to obtain normal distributions and ensure homogeneity of variance for the urinary concentrations. Pearson correlation coefficients were used for an analysis of the relationship between two sets of data with a normal distribution, and the differences between groups were tested via one-way ANOVA when the two sets of data were distributed normally; otherwise, a Mann–Whitney U-test was used to analyze differences between groups. A value of p < 0.05 denoted significance.3. Results and discussion
The measured concentrations (GM, mean, median and range) of 6 mOPs in paired maternal urine and amniotic fluid samples from an e-waste dismantling area in Guangdong province, China are shown in Table 1.| Chlorinated mOPs | Nonchlorinated mOPs | All mOPs | |||||
|---|---|---|---|---|---|---|---|
| BDCIPP | BBOEP | DBP | O + P | DPHP | ∑NCl-mOPsf | Σ6mOPsg | |
| a DR = detection rate. b GM = geometric mean value. c Two effective digits have been used for concentration values. d <LOQ = concentration value lower than the LOQ. e n = the number of samples. f ΣNCl-mOPs represents the sum of urinary concentrations for all four nonchlorinated OP metabolites. g Σ6mOPs represents the sum of urinary concentrations of all six OP metabolites. h Details on the type of samples, including amniotic fluid and maternal urine. | |||||||
| All samples (n = 30) | |||||||
| DRa (%) | 3 | 7 | 93 | 10 | 77 | 97 | 97 |
| Median | <LOQc | <LOQ | <LOQ | <LOQ | 0.6 | 3.24 | 3.58 |
| Min | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.2 | 0.45 |
| Max | 0.83 | 4.3 | 21 | <LOQ | 2.3 | 22 | 22 |
| GMb | 0.23 | 0.066 | 1.9 | 0.039 | 0.34 | 3.2 | 3.6 |
| Mean | 0.25 | 0.27 | 4.7 | 0.046 | 0.70 | 5.7 | 6.1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| By sampling type | |||||||
| Amniotic fluid (n = 15) | |||||||
| DRa (%) | 0 | 0 | 93 | 0 | 60 | 93 | 93 |
| Median | < LOQc | < LOQ | 1.2 | < LOQ | 0.18 | 1.5 | 1.7 |
| Min | < LOQd | < LOQ | < LOQ | < LOQ | < LOQ | 0.2 | 0.45 |
| Max | < LOQ | < LOQ | 13 | < LOQ | 1.1 | 13 | 13 |
| GMb | < LOQ | < LOQ | 1.3 | < LOQ | 0.12 | 1.7 | 2.1 |
| Mean | < LOQ | < LOQ | 2.9 | < LOQ | 0.22 | 3.2 | 3.5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Maternal urine (n = 15) | |||||||
| DRa (%) | 7 | 13 | 93 | 20 | 93 | 100 | 100 |
| Median | <LOQ | <LOQ | 2.2 | <LOQ | 1.2 | 5.1 | 5.4 |
| Min | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 1.3 | 1.4 |
| Max | 0.83 | 4.3 | 21 | <LOQ | 2.3 | 22 | 22 |
| GMb | 0.27 | 0.087 | 2.9 | 0.031 | 0.94 | 5.8 | 6.1 |
| Mean | 0.29 | 0.49 | 6.5 | 0.041 | 1.2 | 8.2 | 8.4 |
3.1 Concentrations in amniotic fluid
The number of amniotic fluid samples with detectable concentrations differed by mOP (DBP: n = 14; DPHP: n = 9; BDCIPP: n = 0; BBOEP: n = 0; O + P: n = 0) (Table 1). Our results provide evidence that in addition to maternal transfer via cord blood, the fetus is also exposed to mOPs through amniotic fluid. As amniotic fluid is the maternal medium that fetuses are physically exposed to, this poses the risk of maternal and fetal transmission and may result in adverse health risks to the fetuses.Across all participants, the GM value of the sum concentration of the 6 mOPs (Σ6mOPs) in amniotic fluid was 2.1 ng ml−1 (Table 1). Among the target mOPs, DBP was the most abundant mOP, with a GM concentration of 1.3 ng ml−1, followed by DPHP (0.12 ng ml−1). It is worth noting that the GM concentration of DBP was one order of magnitude higher than DPHP, indicating higher exposure levels to TnBP (the parent chemical of DBP) or DBP for fetuses. Although no data on mOPs in amniotic fluid was available, a few studies have reported the amniotic fluid concentrations of other environmental chemicals.18,19,23,24 Metabolites of organophosphate pesticides, such as diethyl phosphate, dimethyl phosphate and dimethyl thiophosphate, were detected in amniotic fluid with detection rates (GM value) of 10% (0.31 ng ml−1), 10% (0.32 ng ml−1), and 5% (0.43 ng ml−1), respectively;19 metabolites of organochlorine (e.g., hexachlorobenzene) were frequently detected (66% of amniotic fluid samples) with a median value of 0.023 ng ml−1.18 Phthalate metabolites (range of median values: 0.07 to 0.27 ng ml−1) and PFOS (1.1 ng ml−1) were also detected in amniotic fluid samples.23 In China, the mean concentrations of PFOS and perfluorooctanoic acid (PFOA) in amniotic fluid were reported to be 0.020 and 0.044 ng ml−1, respectively.24 Based on existing studies, the levels of mOPs in amniotic fluid observed in this study (median value: DBP: 1.2 ng ml−1; DPHP: 0.18 ng ml−1) were comparable to or higher than those of other environmental chemicals. However, a combination of the current study and other previous studies18,19,22,23 shows that health outcomes of fetuses that are co-exposed to toxic compounds in amniotic fluid should be further investigated.
3.2 Concentrations in maternal urine
All target mOPs were detected in urine samples, with detection rates of 7–93% (i.e., BDCIPP: 7%; BBOEP: 13%; O + P: 20%; DBP: 93%; and DPHP: 93%) (Table 1). The ubiquity of urinary mOPs observed in this study suggests that pregnant women living in the e-waste dismantling area are widely exposed to OPs. Among all donors, the GM urinary value of Σ6mOPs was 6.1 ng ml−1, and DBP was the most abundant mOP (GM: 2.9 ng ml−1), followed by DPHP (GM: 0.94 ng ml−1), BDCIPP (GM: 0.27 ng ml−1), BBOEP (GM: 0.087 ng ml−1) and O + P (GM: 0.031 ng ml−1). Very few studies have reported urinary levels of mOPs in China.3,25 Feng et al. measured the urinary levels of DPHP (detection rate: 100%) and BDCIPP (17%) in a cohort of pregnant women from Shanghai, China, and GM levels of DPHP and BDCIPP based on detectable concentrations were estimated to be 1.1 and 1.2 ng ml−1, respectively.25 Our results relating to urinary DPHP and BDCIPP levels (based on detectable concentrations) were similar to those found in pregnant women from Shanghai. In an early study, urinary levels of mOPs were reported in general adults living in an e-waste dismantling area; interestingly, our studied pregnant women also had urinary concentrations of BDCIPP, BBOEP, DBP, O + P and DPHP 1 to 7 times higher than the general adults (Table 2) who lived in an e-waste area of the same city, indicating higher exposure to mOPs for pregnant women.3 Furthermore, the median concentration of DBP for pregnant women (2.2 ng ml−1) in this study was one order of magnitude higher than for general adults (0.20 ng ml−1) from Guangzhou and Shenzhen, Guangdong Province of China.26 Hence, pregnant women living in an e-waste area, as a susceptible population in high-risk exposure areas, should be given special attention due to their exposure situation.| Country | Population | N | Sampling locationb | Chlorinated mOPs | Nonchlorinated mOPs | Sampling date | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| BDCIPP | BBOEP | DBP | O + Pc | DPHP | ||||||
| a The number of collected samples. b Represents the location where samples were collected. c “O + P” represents the sum of concentrations of DoCP and DpCP. d “Pregnant women (e-waste)” represents pregnant women from an e-waste dismantling area. e “General adults (e-waste)” represents all general adults from an e-waste dismantling area. f Publication date was shown for these references due to the sampling date being unavailable. g NA = not analyzed (i.e., this chemical was not analyzed in this reference). h NC = not calculated (i.e., this chemical was analyzed, but this value was not calculated in this reference). i The concentrations of mOPs in urine were reported in specific gravity-corrected form in these studies. | ||||||||||
| China | Pregnant women (e-waste)d | 15 | Qingyuan | 0.21 (0.25/0.25) | 0.087 (0.05/0.49) | 2.9 (2.2/6.5) | 0.031 (0.05/0.041) | 0.94 (1.2/1.2) | 2016–2017 | This study |
| China | Pregnant women | 23 | Shanghai | 1.20 (NC/NC) | NA g | NA | NA | 1.10 (NC/NC) | 2015 | 25 |
| United States | Pregnant women | 39 | North Carolina | 1.3 (1.1/NC) | NA | NA | NA | 1.9 (1.6/NC) | 2012–2013 | 26 |
| Canada | Pregnant women | 24 | Ontario | 0.27 (0.26/NC) | 0.38 (<0.08/NC) | NA | 0.64 (0.69/NC) | 2.88 (2.94/NC) | 2010–2012 | 27 |
| United Statesi | Pregnant women | 349 | North Carolina | 1.8 (1.9/NC) | NA | NA | NA | 1.4 (1.3/NC) | 2012–2013 | 45 |
| United Statesi | Pregnant women | 59 | Rhode Island | NC (1.18/NC) | NA | NA | NA | NC (0.93/NC) | 2012–2013 | 46 |
| United Statesi | General adults | 9 | North Carolina | 0.148 (0.083/NC) | NA | NA | NA | 1.074 (0.803/NC) | 2011f | 47 |
| United States | General adults | 53 | North Carolina | 0.37 (NCh/NC) | NA | NA | NA | 1.02 (NC/NC) | 2012–2013 | 28 |
| China | General adults | 29 | Guangzhou | 0.019(<LOQ/0.033) | 0.093 (0.091/0.10) | 0.10 (0.09/0.12) | 0.012 (0.016/0.016) | 0.67 (0.53/2.8) | 2014 | 3 |
| China | General adults (e-waste)e | 121 | Qingyuan | 0.079 (0.11/0.18) | 0.065 (0.072/0.091) | 0.41 (0.20/1.6) | 0.015 (0.016/0.025) | 0.52 (0.53/0.62) | 2014 | 3 |
When comparing urinary concentrations of mOPs in pregnant women between China and other countries (Table 2), urinary levels of BDCIPP for Chinese pregnant women (0.29 ng ml−1) were similar to those found in Canada (0.26 ng ml−1), but much less than those reported in the U.S. (1.1 ng ml−1).26,27 However, the urinary DPHP level (1.2 ng ml−1) observed in this study was consistent with that found in the U.S. (1.6 ng ml−1), but lower than that found in Canada (2.9 ng ml−1).26,27 Similarly, Chinese pregnant women also had lower urinary concentrations of O + P (0.05 ng ml−1) and BBOEP (0.05 ng ml−1) than those reported for pregnant women from Canada (0.69 ng ml−1).27
Furthermore, in the U.S., pregnant women also had higher GM concentrations of urinary BDCIPP (1.3 vs. 0.37 ng ml−1) and DPHP (1.9 vs. 1.0 ng ml−1) than those observed in general adults.26,28 Further, Hoffman et al. found that GM concentrations were 3 to 10 times greater in pregnant women than those reported in general adults, which is consistent with our results that found that pregnant women were more exposed to OPs than general adults in the same location.26 Interestingly, women in a pregnancy and nutrition cohort were also found to have higher levels of PBDEs in their breast milk than women in other similarly timed studies in U.S. cohorts29. The high presence of these environmental chemicals and their metabolites in pregnant women may indicate that they are more exposed to flame retardants from different consumer products and the environment. The pregnant women in this study were assessed as having been hospitalized for 7–11 days after caesarean section. At the same time, samples collected for this study and studies in other countries were almost all from hospitals. Therefore, these pregnant women are hospitalized. Recently, another of our studies found that the median urinary levels of mOPs in inpatient infants were one order of magnitude higher (p < 0.01) than those observed in outpatient infants, suggesting unexplained higher exposure during hospitalization.30
However, no study has reported OP levels in indoor dust (or air) and medical materials in hospitals. Huygh et al. studied serum and urine samples of adult patients and found that these patients are continuously exposed to phthalates, such as di(2-ethylhexyl)phthalate (DEHP), as well as to BPA.31 Plastic-containing medical devices may be the main source of plasticizer exposure for inpatients, including pregnant women near the time of maternity.31 Thus, further studies are needed to examine the residue levels of OPs in hospital decoration materials, medical apparatus and instruments. Furthermore, pregnant women stay in an indoor environment for a long time, especially when they are about to give birth. Previous studies have examined the occurrence of 12 OPs in indoor dust in four micro-environments of southern China; indoor dust OPs were widely determined and their concentrations in an e-waste area (median: 25 μg g−1) were significantly higher than in a rural non-e-waste area (7.48–11.0 μg g−1).20,32 Therefore, exposure to indoor dust may also cause higher OP exposure for pregnant women than for general adults. Also, differences in excretion rates and kidney function during pregnancy may explain the higher mOP levels observed in the pregnant cohort relative to other non-pregnant cohorts.26
3.3 Correlation of mOPs between amniotic fluid and maternal urine
In this study, all 6 mOPs were detected in maternal urine while only 2 mOPs were detected in amniotic fluid; the discussion in this section is mainly focused on these two substances (i.e., DBP and DPHP). Among all mOPs, DBP and DPHP were widely detected in maternal urine and amniotic fluid. The detection rate of DBP was 93% in both matrices, while the detection rate of DPHP in maternal urine was 93%, slightly higher than that in amniotic fluid (60%). Coincidentally, the mOPs that are not detected in amniotic fluid, including BDCIPP, BBOEP, and O + P, also had low detection rates in urine (DR: 7–27%). This result indicates that chemical exposure exhibits similarity or consistency with respect to the detection rates and composition profiles between maternal urine and amniotic fluid from the same individual. This further illustrates that there is correlation between maternal exposure and fetal exposure to environmental chemicals.However, the residue levels of mOPs in these matrices were different between amniotic fluid and maternal urine. The urinary concentrations of DPHP (GM: 0.94 ng ml−1) and DBP (2.9 ng ml−1) were significantly higher than those in amniotic fluid (GM: 0.12 ng ml−1 for DPHP; 1.3 ng ml−1 for DBP) (p < 0.05). The lower concentrations (Table 1) of mOPs in amniotic fluid might be because the placental barrier has a limited filtering effect.19 In addition, the differences in elimination capacities between pregnant women and fetuses may also cause the lower concentrations of mOPs in amniotic fluid compared to those in maternal urine.
The Pearson's correlation coefficient was used to estimate the correlation of DBP or DPHP concentrations between maternal urine and amniotic fluid samples. No significant correlation was observed between paired maternal urine and amniotic fluid samples for the concentrations of DBP (r = −0.508, p > 0.05) and DPHP (r = 0.097, p > 0.05); this may be because of the small data sample size. Previous studies reported associations related to the concentrations of MBP and MiBP between amniotic fluid and maternal urine samples,33,34 which suggests that further studies are needed to clarify the correlation related to mOPs between amniotic fluid and maternal urine.
3.4 Human exposure to OPs and a risk assessment
The estimated daily intake (EDI) of OPs by pregnant women was calculated according to the following equation, which was adopted from previous studies with slight modifications.35 Parameters used for the calculations of EDIs are shown in Table S2.† The results for EDIs are shown in Table 3. The equation used is as follows (eqn (1)):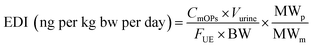 | (1) |
| TnBP | TPHPHLMa | TPHPS9b | |
|---|---|---|---|
| a Estimated daily intakes were calculated using FUE values of TPHP in a human liver microsome system. b Estimated daily intakes were calculated using FUE values of TPHP in a human S9 microsome system. | |||
| RfD | 2400 | 7000 | 7000 |
| GM | 404 | 273 | 613 |
| Median | 307 | 334 | 751 |
| Mean | 909 | 344 | 773 |
| Range | 7.0–3000 | 14–680 | 33–1530 |
| Over standard rate | 13% | 0% | 0% |
In this study, the GM (range) values of EDIHLM-TPHP and EDIS9-TPHP were estimated to be 273 (14 to 680) and 613 (33 to 1530) ng per kg bw per day, respectively; and the GM (range) value for EDITnBP was 404 (7.0 to 3000) ng per kg bw per day. In order to assess the exposure risk related to OPs for our pregnant women, we estimated the percentage of donors who had EDIs over the corresponding reference doses (RfDs). The RfDs were chosen from previous studies: 2400 and 7000 ng per kg bw per day for TnBP and TPHP, respectively.38 The GM and maximum EDI values for EDIHLM-TPHP and EDIS9-TPHP in pregnant women were all one magnitude order lower than the RfDs from the present study, indicating that the risk from TPHP exposure was at a low level. Although the GM value for EDITnBP obtained in the present study was below its corresponding RfD, 13% of pregnant women had an EDITnBP value higher than the RfD value for TnBP, indicating a potential health risk at the current levels of TnBP exposure for pregnant women living in the e-waste dismantling area.
The results suggested a need for additional studies on the exposure risks related to TnBP for humans. In a laboratory animal study, TnBP can reduce the levels of antioxidant enzymes and heat shock protein related genes in Asian freshwater clams.41 TnBP exposure resulted in a significant increase in the mRNA level of elavl3 in zebrafish larvae, which might be regarded as part of a compensatory response.42 Further, neurobehavioral responses are well conserved among vertebrates; as fetuses are vulnerable to OP exposure, an assessment of the risks of OP exposure during early development should be highly emphasized in future studies42. Furthermore, TnBP was also the major OP among those monitored in marine and freshwater fish, with total levels ranging from 1.4 to 6000 ng g−1 lipid weight (lw).1 Higher concentrations of TnBP were also found in fish collected from bodies of water in the Pearl River Delta region of southern China, over a range of 43.9–2946 ng g−1 lw.43 The accumulation of TnBP can be attributed to its high environmental contamination levels as the production volumes and applications of TnBP have increased over time.1 A new report suggests that TnBP is the dominant non-halogenated OP detected in newly decorated houses in China, implying that TnBP was commonly used in building and decoration materials in China.44 Thus, more attention should be paid to OP release from OP-containing electronic products in e-waste recycling areas of China, especially TnBP.
Although this is the first time gaining insight into fetal exposure to OPs in an e-waste dismantling area of China, our results should be interpreted in the context of several limitations. First, the number of samples was small, and the patterns of exposure could be different across pregnant women in the same location. In addition, this study estimated the concentrations of external exposure through the levels of internal exposure, so individual metabolic capacity will also affect EDI calculations. Nevertheless, this pilot study is an important step in understanding maternal and fetal OP exposure. Second, estimates of the molar fractions (i.e., FUE) of OPs converted into their metabolites were based on in vitro studies (in HLM and S9 fraction systems), which may introduce uncertainty into the excretion rates of OP metabolites.39 Third, controversies surround the issue of reference doses and threshold concentrations of OPs; therefore, these values should be considered tentative.
4. Conclusions
In summary, this study provided novel information on fetal exposure to OPs (or mOPs) through the biomonitoring of mOPs in amniotic fluid. Pregnant women and their fetuses, living in an e-waste dismantling area, were widely exposed to OPs. DBP and DPHP were detected at high levels in maternal urine as well as in paired amniotic fluid, with GM values much higher than those in general adults living in the same location and in other Chinese cities. A risk assessment indicated that 13% of the investigated pregnant women had EDITnBP values higher than the corresponding RfD, demonstrating high exposure levels to TnBP, which needs more attention.Conflicts of interest
The authors declare no competing interests.Acknowledgements
The Natural Science Foundation of China (No. 21677184 and No. 41877375), the Pearl River S&T Nova Program of Guangzhou, the Natural Science Foundation of Guangdong Province, China (No. 2015A030313869), and Shenzhen Government Research Projects (No. JCYJ20160428143348745) are acknowledged for their partial research support. The present study was also supported by the Guangzhou Key Laboratory of Environmental Exposure and Health (No. GZKLEEH201606); and the State Key Laboratory of Environmental Chemistry and Ecotoxicology, the Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences (No. KF2016-25). We gratefully acknowledge the donors who contributed samples for this study.References
- R. Hou, C. Huang, K. Rao, Y. Xu and Z. Wang, Characterized in vitro metabolism kinetics of alkyl organophosphate flame retardants in fish liver and intestinal microsomes, Environ. Sci. Technol., 2018, 52, 5 Search PubMed.
- I. V. D. Veen and J. D. Boer, Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis, Chemosphere, 2012, 88, 1119–1153 CrossRef.
- S. Y. Lu, Y. X. Li, T. Zhang, D. Cai, J. J. Ruan, M. Z. Huang, L. Wang, J. Q. Zhang and R. L. Qiu, Effect of e-waste recycling on urinary metabolites of organophosphate flame retardants and plasticizers and their association with oxidative stress, Environ. Sci. Technol., 2017, 51, 4 CrossRef.
- E. Cequier, A. C. Ionas, A. Covaci, R. M. Marcé, G. Becher and C. Thomsen, Occurrence of a broad range of legacy and emerging flame retardants in indoor environments in Norway, Environ. Sci. Technol., 2014, 48, 6827–6835 CrossRef CAS.
- J. Li, N. Yu, B. Zhang, L. Jin, M. Li, M. Hu, X. Zhang, S. Wei and H. Yu, Occurrence of organophosphate flame retardants in drinking water from China, Water Res., 2014, 54, 53–61 CrossRef.
- X. Zhang, W. Zou, L. Mu, Y. Chen, C. Ren, X. Hu and Q. Zhou, Rice ingestion is a major pathway for human exposure to organophosphate flame retardants (OPFRs) in China, J. Hazard. Mater., 2016, 318, 686–693 CrossRef CAS PubMed.
- Z. Cao, F. Xu, A. Covaci, M. Wu, H. Wang, G. Yu, B. Wang, S. Deng, J. Huang and X. Wang, Distribution patterns of brominated, chlorinated, and phosphorus flame retardants with particle size in indoor and outdoor dust and implications for human exposure, Environ. Sci. Technol., 2014, 48, 8839–8846 CrossRef CAS.
- M. Tian, S. J. Chen, J. Wang, X. B. Zheng, X. J. Luo and B. X. Mai, Brominated flame retardants in the atmosphere of e-waste and rural sites in southern China: seasonal variation, temperature dependence, and gas-particle partitioning, Environ. Sci. Technol., 2011, 45, 8819–8825 CrossRef CAS.
- W. J. Deng, J. S. Zheng, X. H. Bi, J. M. Fu and M. H. Wong, Distribution of PBDEs in air particles from an electronic waste recycling site compared with Guangzhou and Hong Kong, South China, Environ. Int., 2007, 33, 1063–1069 CrossRef CAS.
- D. Chen, X. Bi, J. Zhao, L. Chen, J. Tan, B. Mai, G. Sheng, J. Fu and M. Wong, Pollution characterization and diurnal variation of PBDEs in the atmosphere of an e-waste dismantling region, Environ. Pollut., 2009, 157, 1051–1057 CrossRef CAS PubMed.
- F. Li, L. Cao, X. Li, N. Li, Z. Wang and H. Wu, Affinities of organophosphate flame retardants to tumor suppressor gene p53: an integrated in vitro and in silico study, Toxicol. Lett., 2015, 232, 533–541 CrossRef CAS PubMed.
- S. Kim, J. Jung, I. Lee, D. Jung, H. Youn and K. Choi, Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines, Aquat. Toxicol., 2015, 160, 188–196 CrossRef CAS PubMed.
- I. Kozikowska, K. Miszczuk and R. Stawarz, Mercury concentrations in human placenta, umbilical cord, cord blood and amniotic fluid and their relations with body parameters of newborns, Environ. Pollut., 2013, 182, 256–262 CrossRef CAS.
- K. Jaraczewska, J. Lulek, A. Covaci, S. Voorspoels, A. Kaluba-Skotarczak, K. Drews and P. Schepens, Distribution of polychlorinated biphenyls, organochlorine pesticides and polybrominated diphenyl ethers in human umbilical cord serum, maternal serum and milk from Wielkopolska region, Poland, Sci. Total Environ., 2006, 372, 20–31 CrossRef CAS.
- M. Frederiksen, C. Thomsen, M. FrøShaug, K. Vorkamp, M. Thomsen, G. Becher and L. E. Knudsen, Polybrominated diphenyl ethers in paired samples of maternal and umbilical cord blood plasma and associations with house dust in a Danish cohort, Int. J. Hyg. Environ. Health, 2010, 213, 233–242 CrossRef CAS PubMed.
- X. Zhang, X. Li, Y. Jing, X. Fang, X. Zhang, B. Lei and Y. Yu, Transplacental transfer of polycyclic aromatic hydrocarbons in paired samples of maternal serum, umbilical cord serum, and placenta in Shanghai, China, Environ. Pollut., 2017, 222, 267–275 CrossRef CAS PubMed.
- G. Toft, A. G. J. Bo, J. P. Bonde, B. Nørgaard-Pedersen, D. M. Hougaard, A. Cohen, C. H. Lindh, R. Ivell, R. Anand-Ivell and M. S. Lindhard, Perfluorooctane Sulfonate Concentrations in Amniotic Fluid, Biomarkers of Fetal Leydig Cell Function, and Cryptorchidism and Hypospadias in Danish Boys (1980–1996), Environ. Health Perspect., 2016, 124, 151–156 CAS.
- O. P. Luzardo, V. Mahtani, J. M. Troyano, d. l. R. M. Alvarez, A. I. Padilla-Pérez, M. Zumbado, M. Almeida, G. Burillo-Putze, C. Boada and L. D. Boada, Determinants of organochlorine levels detectable in the amniotic fluid of women from Tenerife Island (Canary Islands, Spain), Environ. Res., 2009, 109, 607–613 CrossRef CAS.
- A. Bradman, D. B. Barr, T. Drumheller, C. Curry and B. Eskenazi, Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: A validation study, Environ. Health Perspect., 2003, 111, 1779–1782 CrossRef CAS.
- C. T. He, J. Zheng, L. Qiao, S. J. Chen, J. Z. Yang, J. G. Yuan, Z. Y. Yang and B. X. Mai, Occurrence of organophosphorus flame retardants in indoor dust in multiple microenvironments of southern China and implications for human exposure, Chemosphere, 2015, 133, 47–52 CrossRef CAS.
- T. Zhang, J. Xue, C. Z. Gao, R. L. Qiu, Y. X. Li, X. Li, M. Z. Huang and K. Kannan, Urinary concentrations of bisphenols and their association with biomarkers of oxidative stress in people living near e-waste recycling facilities in China, Environ. Sci. Technol., 2016, 50, 4045 CrossRef CAS PubMed.
- R. W. Hornung and L. D. Reed, Estimation of Average Concentration in the Presence of Nondetectable Values, App. Occup. Envron. Hyg., 1990, 5, 46–51 CrossRef CAS.
- M. S. Jensen, B. Nørgaardpedersen, G. Toft, D. M. Hougaard, J. P. Bonde, A. Cohen, A. M. Thulstrup, R. Ivell, R. Anandivell and C. H. Lindh, Phthalates and perfluorooctanesulfonic acid in human amniotic fluid: temporal trends and timing of amniocentesis in pregnancy, Environ. Health Perspect., 2012, 120, 897–903 CrossRef CAS.
- T. Zhang and X. Qin, Assessment of fetal exposure and maternal elimination of perfluoroalkyl substances, Environ. Sci.: Processes Impacts, 2014, 16, 1878–1881 RSC.
- L. Feng, F. Ouyang, L. Liu, X. Wang, X. Wang, Y. J. Li, A. Murtha, H. Shen, J. Zhang and J. J. Zhang, Levels of urinary metabolites of organophosphate flame retardants, TDCIPP, and TPHP, in pregnant women in Shanghai, J. Environ. Public Health, 2016, 1–7 Search PubMed.
- K. Hoffman, J. L. Daniels and H. M. Stapleton, Urinary metabolites of organophosphate flame retardants and their variability in pregnant women, Environ. Int., 2014, 63, 169–172 CrossRef CAS PubMed.
- I. Kosarac, C. Kubwabo and W. G. Foster, Quantitative determination of nine urinary metabolites of organophosphate flame retardants using solid phase extraction and ultra performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS), J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2016, 1014, 24–30 CrossRef CAS.
- K. Hoffman, S. Garantziotis, L. S. Birnbaum and H. M. Stapleton, Monitoring indoor exposure to organophosphate flame retardants: hand wipes and house dust, Environ. Health Perspect., 2015, 123, 160–165 CrossRef CAS.
- J. L. Daniels, I. Pan, R. Jones, S. Anderson, D. G. P. Jr, L. L. Needham and A. Sjödin, Individual Characteristics Associated with PBDE levels in U.S. human milk samples, Environ. Health Perspect., 2010, 118, 155–160 CrossRef CAS PubMed.
- B. Zhang, S. Y. Lu, M. Z. Huang, M. Z. Zhou, Z. Q. Zhou, H. C. Zheng, Y. C. Jiang, X. Y. Bai and T. Zhang, Urinary metabolites of organophosphate flame retardants in 0–5-year-old children: potential exposure risk for inpatients and home-stay infants, Environ. Pollut., 2018, 243, 318–325 CrossRef CAS PubMed.
- J. Huygh, K. Clotman, G. Malarvannan, A. Covaci, T. Schepens, W. Verbrugghe, E. Dirinck, G. L. Van and P. G. Jorens, Considerable exposure to the endocrine disrupting chemicals phthalates and bisphenol-A in intensive care unit (ICU) patients, Environ. Int., 2015, 81, 64–72 CrossRef CAS.
- C. M. Butt, M. L. Diamond, J. Truong, M. G. Ikonomou and A. F. ter Schure, Spatial distribution of polybrominated diphenyl ethers in southern Ontario as measured in indoor and outdoor window organic films, Environ. Sci. Technol., 2004, 38, 724–731 CrossRef CAS PubMed.
- P. C. Huang, P. L. Kuo, Y. Y. Chou, S. J. Lin and C. C. Lee, Association between prenatal exposure to phthalates and the health of newborns, Environ. Int., 2009, 35, 14–20 CrossRef CAS.
- M. Wittassek, J. Angerer, M. Kolossagehring, S. D. Schäfer, W. Klockenbusch, L. Dobler, A. K. Günsel, A. Müller and G. A. Wiesmüller, Fetal exposure to phthalates – a pilot study, Int. J. Hyg. Environ. Health, 2009, 212, 492–498 CrossRef CAS PubMed.
- H. Fromme, T. Lahrz, M. Kraft, L. Fembacher, C. Mach, S. Dietrich, R. Burkardt, W. Völkel and T. Göen, Organophosphate flame retardants and plasticizers in the air and dust in German daycare centers and human biomonitoring in visiting children (LUPE 3), Environ. Int., 2014, 71, 158–163 CrossRef CAS PubMed.
- J. Valentin, Annals of the ICRP. Basic Anatomical and Physiological Data for Use in Radiological Protection: Reference Values, 2002 Search PubMed.
- K. Hoffman, L. Gearhart-Serna, M. Lorber, T. F. Webster and H. M. Stapleton, Estimated tris(1,3-dichloro-2-propyl) phosphate exposure levels for U.S. infants suggest potential health risks, Environ. Sci. Technol. Lett., 2017, 4, 8 Search PubMed.
- D. E. N. Van, A. C. Dirtu, H. Neels and A. Covaci, Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust, Environ. Int., 2011, 37, 454–461 CrossRef PubMed.
- D. E. N. Van, W. Maho, C. Erratico, H. Neels and A. Covaci, First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions, Toxicol. Lett., 2013, 223, 9–15 CrossRef PubMed.
- T. Suzuki, K. Sasaki, M. Takeda and M. Uchiyama, Metabolism of tributyl phosphate in male rats, J. Agric. Food Chem., 1984, 32, 603–610 CrossRef CAS.
- S. Yan, H. Wu, J. Qin, J. Zha and Z. Wang, Halogen-free organophosphorus flame retardants caused oxidative stress and multixenobiotic resistance in Asian freshwater clams (Corbicula fluminea), Environ. Pollut., 2017, 225, 559–568 CrossRef CAS PubMed.
- L. Sun, W. Xu, T. Peng, H. Chen, L. Ren, H. Tan, D. Xiao, H. Qian and Z. Fu, Developmental exposure of zebrafish larvae to organophosphate flame retardants causes neurotoxicity, Neurotoxicol. Teratol., 2016, 55, 16–22 CrossRef CAS.
- Y. Ma, K. Cui, F. Zeng, J. Wen, H. Liu, F. Zhu, G. Ouyang, T. Luan and Z. Zeng, Microwave-assisted extraction combined with gel permeation chromatography and silica gel cleanup followed by gas chromatography–mass spectrometry for the determination of organophosphorus flame retardants and plasticizers in biological samples, Anal. Chim. Acta, 2013, 786, 47–53 CrossRef CAS.
- Y. Wang, M. Hou, Q. Zhang, X. Wu, H. Zhao, Q. Xie and J. Chen, Organophosphorus flame retardants and plasticizers in building and decoration materials and their potential burdens in newly decorated houses in China, Environ. Sci. Technol., 2017, 51, 19 Search PubMed.
- K. Hoffman, A. Lorenzo, C. M. Butt, L. Adair, A. H. Herring, H. M. Stapleton and J. L. Daniels, Predictors of urinary flame retardant concentration among pregnant women, Environ. Int., 2016, 98, 96–101 CrossRef PubMed.
- M. E. Romano, N. L. Hawley, M. Eliot, A. M. Calafat, N. K. Jayatilaka, K. Kelsey, S. Mcgarvey, M. G. Phipps, D. A. Savitz and E. F. Werner, Variability and predictors of urinary concentrations of organophosphate flame retardant metabolites among pregnant women in Rhode Island, Environ. Health, 2017, 16, 40 CrossRef PubMed.
- E. M. Cooper, A. Covaci, A. L. N. V. Nuijs, T. F. Webster and H. M. Stapleton, Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography–tandem mass spectrometry, Anal. Bioanal. Chem., 2011, 401, 2123–2132 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8em00389k |
| ‡ These authors contributed to this work equally. |
| This journal is © The Royal Society of Chemistry 2019 |
