Cable-like Ru/WNO@C nanowires for simultaneous high-efficiency hydrogen evolution and low-energy consumption chlor-alkali electrolysis†
Abstract
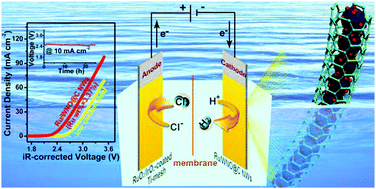
The rational design of high-efficiency and stable hydrogen evolution electrocatalysts under the condition of strong alkali is the key issue for the combination of hydrogen production with low-energy consumption chlor-alkali electrolysis. Herein, ultra-small Ru nanoclusters anchored on WNO nanowires covered by few-layer N-doped carbon (named Ru/WNO@C) were synthesized through a simple pyrolysis method. We demonstrate a comprehensive understanding of the hydrogen evolution reaction (HER) performance of such cable-like Ru/WNO@C electrocatalysts by combining experimental and computational techniques. The optimal catalyst Ru/WNO@C (Ru wt% = 3.37%) delivers a record-low overpotential of 2 mV at a current density of 10 mA cm−2, a low Tafel slope of 33 mV dec−1, a high mass activity of 4095.6 mA mg−1 at an overpotential of 50 mV, and long-term durability in 1 M KOH. The superior HER activity of Ru/WNO@C is revealed to be caused by two factors using density functional theory (DFT) calculations: a moderate H adsorption free energy (ΔGH* = −0.21 eV) and a rather low water dissociation barrier (ΔGB = 0.27 eV). Specifically, Ru/WNO@C (Ru wt% = 3.37%) shows more remarkable HER performance than industrial low carbon steel under a simulated chlor-alkali electrolyte at 90 °C, making it an efficient cathode candidate applied in chlor-alkali electrolysis. Finally, a homemade ionic membrane electrolyzer with a Ru/WNO@C (Ru wt% = 3.37%) (−)//RuO2/IrO2-coated Ti-mesh (+) couple presents a low cell voltage of 2.48 V at a current density of 10 mA cm−2, which is 320 mV lower than the value for the low carbon steel (−)//RuO2/IrO2-coated Ti-mesh (+) (2.8 V) couple, exhibiting robust stability for 25 h. This work provides a meaningful reference for the design and fabrication of efficient and stable alkaline HER catalysts, and realizes high-efficiency hydrogen production and low-energy consumption chlor-alkali electrolysis at the same time.


 Please wait while we load your content...
Please wait while we load your content...