Si photocathode with Ag-supported dendritic Cu catalyst for CO2 reduction†
Abstract
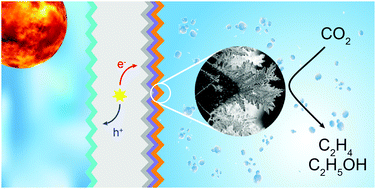
Si photocathodes integrated with Ag-supported dendritic Cu catalysts are used to perform light-driven reduction of CO2 to C2 and C3 products in aqueous solution. A back illumination geometry with an n-type Si absorber was used to permit the use of absorbing metallic catalysts. Selective carrier collection was accomplished by a p+ implantation on the illumination side and an n+ implantation followed by atomic layer deposition of TiO2 on the electrolyte site. The Ag-supported dendritic Cu CO2 reduction catalyst was formed by evaporation of Ag followed by high-rate electrodeposition of Cu to form a high surface area structure. Under simulated 1 sun illumination in 0.1 M CsHCO3 saturated with CO2, the photovoltage generated by the Si (∼600 mV) enables C2 and C3 products to be produced at −0.4 vs. RHE. Texturing of both sides of the Si increases the light-limited current density, due to reduced reflection on the illumination side, and also deceases the onset potential. Under simulated diurnal illumination conditions photocathodes maintain over 60% faradaic efficiency to hydrocarbon and oxygenate products (mainly ethylene, ethanol, propanol) for several days. After 10 days of testing, contamination from the counter electrode is observed, which causes an increase in hydrogen production. This effect is mitigated by a regeneration procedure which restores the original catalyst selectivity. A tandem, self-powered CO2 reduction device was formed by coupling a Si photocathode with two series-connected semitransparent CH3NH3PbI3 perovskite solar cells, achieving an efficiency for the conversion of sunlight to hydrocarbons and oxygenates of 1.5% (3.5% for all products).


 Please wait while we load your content...
Please wait while we load your content...