Highly efficient prismatic perovskite solar cells†
Abstract
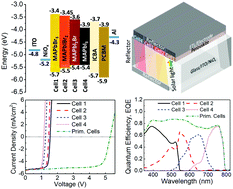
Lead halide perovskites, such as methylammonium lead iodide (MAPbI3) can reach near 100% internal quantum efficiency in single solar cells, but they still encounter significant thermodynamic losses in photon energy to offset device photovoltage and performance. Herein, a novel prismatic perovskite solar cell with light trapping configuration, namely, Prim PVSC, is designed to mitigate such losses in devices, through modulating the pathway of light inside series cells, wherein incident high-to-low energy photons are separately captured by four horizontally aligned MAPbIxBr3−x subcells with varied bandgaps. This newly designed PVSC has remarkably led to a record open circuit voltage of 5.3 V in four series devices and power conversion efficiency of 21.3%, which could provide a new way to break the performance bottleneck of perovskites. Practically, this type of device architecture could also be applied in flexible modules for large-area application.


 Please wait while we load your content...
Please wait while we load your content...