Levelized cost of CO2 mitigation from hydrogen production routes†
B.
Parkinson
 *a,
P.
Balcombe
*a,
P.
Balcombe
 ab,
J. F.
Speirs
ab,
J. F.
Speirs
 bc,
A. D.
Hawkes
bc,
A. D.
Hawkes
 ab and
K.
Hellgardt
a
ab and
K.
Hellgardt
a
aDepartment of Chemical Engineering, Imperial College London, SW7 2AZ, UK. E-mail: b.parkinson17@imperial.ac.uk
bSustainable Gas Institute, Imperial College London, SW7 1NA, UK
cDepartment of Earth Science and Engineering, Imperial College London, SW7 2BP, UK
First published on 17th November 2018
Abstract
Different technologies produce hydrogen with varying cost and carbon footprints over the entire resource supply chain and manufacturing steps. This paper examines the relative costs of carbon mitigation from a life cycle perspective for 12 different hydrogen production techniques using fossil fuels, nuclear energy and renewable sources by technology substitution. Production costs and life cycle emissions are parameterized and re-estimated from currently available assessments to produce robust ranges to describe uncertainties for each technology. Hydrogen production routes are then compared using a combination of metrics, levelized cost of carbon mitigation and the proportional decarbonization benchmarked against steam methane reforming, to provide a clearer picture of the relative merits of various hydrogen production pathways, the limitations of technologies and the research challenges that need to be addressed for cost-effective decarbonization pathways. The results show that there is a trade-off between the cost of mitigation and the proportion of decarbonization achieved. The most cost-effective methods of decarbonization still utilize fossil feedstocks due to their low cost of extraction and processing, but only offer moderate decarbonisation levels due to previous underestimations of supply chain emissions contributions. Methane pyrolysis may be the most cost-effective short-term abatement solution, but its emissions reduction performance is heavily dependent on managing supply chain emissions whilst cost effectiveness is governed by the price of solid carbon. Renewable electrolytic routes offer significantly higher emissions reductions, but production routes are more complex than those that utilise naturally-occurring energy-dense fuels and hydrogen costs are high at modest renewable energy capacity factors. Nuclear routes are highly cost-effective mitigation options, but could suffer from regionally varied perceptions of safety and concerns regarding proliferation and the available data lacks depth and transparency. Better-performing fossil-based hydrogen production technologies with lower decarbonization fractions will be required to minimise the total cost of decarbonization but may not be commensurate with ambitious climate targets.
Broader contextGlobal energy consumption is expected to continue to grow, requiring decarbonized energy vectors for end use applications. Hydrogen as an energy carrier is one possible solution. However, it is relatively expensive and not necessarily low-carbon with its method of production largely determining costs and climate impacts. In addition, comparisons between fossil-routes and renewable based hydrogen often misuse cost and emissions data to push policy makers and public opinion further along the polarizing debate of the role of fossil fuels in a low-carbon energy system. In this context, identifying robust ranges of key parameters to describe the uncertainties for each technology, and combining cradle-to-gate emissions and cost assessments provides a clearer picture of the relative merits of various hydrogen production pathways. The analysis shows that the levelized cost of carbon mitigation from hydrogen production by technology substitution varies by over an order of magnitude, with large emissions contributions originating from supply chains. There is broadly a trade-off between the cost of mitigation and proportion of decarbonization achieved, with cheaper options not achieving decarbonisation fractions commensurate with ambitious global climate targets. |
1. Introduction
The drive to decarbonise is gathering pace as nations commit to climate change targets,1 which will require innovative and socially palatable solutions. Low-carbon hydrogen may be one solution, given its lack of direct CO2 emissions and suitability across energy services and chemicals production.2 Globally, approximately 60 Mt of hydrogen is produced annually3 for the production of nitrogen-based fertilizers (49% of global H2 production), hydro-treatment in petroleum refining (37%), methanol (8%) and other smaller markets (6%) including for use in stationary fuel cells and fuel cell vehicles.4–6 However, 96% of hydrogen is produced by technologies involving the reforming of fossil fuel feedstocks, resulting in high CO2 emissions (natural gas contributes 49%, liquid hydrocarbons 29%, and coal 18%).5 The remaining 4% is produced by electrolysis technologies, which are only low-carbon if powered by low-carbon electricity and embodied emissions of the generation technologies are also low.7 Due to low regional gas and coal prices, and high resource availability, hydrogen production in the short-to-medium term is likely to continue to rely heavily on fossil fuels.8There are many options to produce low-carbon hydrogen, but each option exhibits different levels of decarbonization, whilst there are also many barriers to production through alternative routes, not least scale and costs.9 The challenge of transitioning to more sustainable hydrogen production routes will require focus on the highest return on investment to minimise the cost of decarbonization.10 However, both life cycle emissions and costs may be highly variable and these estimates are poorly bounded within current studies.
Numerous studies have investigated the merits of alternative H2 production technologies through comparative assessments or overall metrics. Ewan et al.11 developed an overall Figure of Merit (FOM) assessment, which evaluated 14 different hydrogen technologies on CO2 emissions intensity, power density, land use and cost. A clear division between low energy density renewable technologies and those associated with high energy densities was shown, with a significantly higher FOM for the later grouping.11 Acar et al.12 compared 8 different hydrogen production technologies for environmental impact, cost and exergy efficiencies. Their study used a social cost of carbon to apply economic consequences to carbon externalities to investigate the relationship between environmental and economic factors. This study was further extended by Dincer et al.,13 comparing 19 different hydrogen production methods using renewable and non-renewable sources for environmental impact, cost, energy and exergy efficiencies. The study found hybrid nuclear thermochemical cycles to be the most promising candidate to produce hydrogen with low environmental and cost impacts.13 Machhammer et al.14 compared two conventional technologies, coal gasification and SMR, with four emerging technologies, metal-oxide cycles, water electrolysis, biomass gasification and methane pyrolysis on cost and carbon footprint. The ‘econological’ result yielded methane pyrolysis to be the most promising technology for low-cost and low carbon footprint hydrogen.14 Speirs et al.15 assessed the evidence of costs and greenhouse gas (GHG) emissions of 9 hydrogen production routes and found that supply chain emissions associated with supposedly low carbon routes were non-negligible and highly variable across regions, processes and estimation assumptions.
Of the different frameworks presented in the literature for comparing hydrogen production technologies, none have directly compared the total costs of production to CO2 emissions from a life cycle perspective for evaluating costs of CO2 mitigation, whilst many include only a subset of available feedstocks and production routes. Additionally, none of the comparison studies include robust ranges of both variations in cost and emissions data available in the literature. Therefore, the goal of this study is to identify the most cost-effective technologies for reducing the carbon intensity of hydrogen production in terms of cost per tonne of carbon dioxide emitted ($ t−1 CO2e) by conducting a detailed engineering, environmental and cost assessment of a full suite of hydrogen production routes and feedstocks. Key parameters for each hydrogen supply chain are quantitatively assessed to develop robust ranges of emissions and costs, as well as providing insight into the best opportunities for carbon reductions. This output is used to estimate the levelized cost of emissions mitigation and the degree of decarbonisation achievable to provide critical guidance on future resource allocation, priorities and trajectory for hydrogen development pathways. The technical and financial hurdles to implementation are discussed and recommendations for future resource allocation are made. This study is the first comprehensive combined supply chain and point source assessment of carbon mitigation costs for the breadth of hydrogen production processes and feedstocks considered. It aims to serve as a valuable source of data and discussion for engineers, academics and policy makers alike to provide a clearer picture of the relative decarbonization merits of hydrogen production pathways.
This study focusses on technologies capable of large-scale production commensurate with current hydrogen demands. Technologies for hydrogen production fall into four categories: (i) thermochemical, (ii) electrochemical, (iii), photo-biological and (iv) photo-electrochemical.16 However, photo-biological and photo-electrochemical methods are not discussed here as these technologies are currently limited by relatively slow production rates, early stages of commercial development and a range of technical issues to overcome before practical applications can be considered (see ref. 17 and 18 for further information). The technologies considered are shown in Table 1. The substitution of a hydrogen production technology with a less CO2-intensive technology assumes it is capable of meeting the hypothetical scale and load profile required. It should also be noted the varying stages of technological readiness of each of the pathways considered in this analysis. Other natural gas reforming routes such as auto-thermal reforming and partial oxidation have not been considered here. Whilst these technologies have different processing steps and reaction pathways resulting in different costs and emissions, they ultimately represent reforming of natural gas. A more detailed comparison of the hydrogen production pathways from natural gas is the subject of future work.
| No. | Technology name | Energy vector | Input material | TRL19,20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
|---|---|---|---|---|
| a Bold text is used throughout to highlight the lower TRL of the production route where comparisons are made. | ||||
| 1 | Steam methane reforming | Thermal | Natural gas | 9 |
| 2 | Steam methane reforming with CCS | Natural gas | 7–8 | |
| 3 | Coal gasification | Coal | 9 | |
| 4 | Coal gasification with CCS | Coal | 6–7 | |
| 5 | Methane pyrolysis | Natural gas | 3–5 | |
| 6 | Biomass gasification | Biomass | 5–6 | |
| 7 | Biomass gasification with CCS | Biomass | 3–5 | |
| 8 | Electrolysis – wind | Electrical | Water | 9 |
| 9 | Electrolysis – solar | Water | 9 | |
| 10 | Electrolysis – nuclear | Water | 9 | |
| 11 | Thermochemical water splitting (S–I) cycle | Electrical + thermal | Water | 3–4 |
| 12 | Thermochemical water splitting (Cu–Cl) cycle | Water | 3–4 | |
2. Methodology
To achieve the above aims, the study is carried out in five steps:(1) A review of recent literature for hydrogen production costs and life cycle emissions;
(2) Define and quantify the key parameters that influence Life Cycle Emissions (LCE) and the Levelized Cost of Hydrogen (LCOH) for each technology;
(3) Re-estimate total LCE and LCOH for each technology based on key parameter ranges identified;
(4) Develop a framework to evaluate technologies based on Levelized Cost of Carbon Mitigation (LCCM) and proportional emissions reductions relative to industry standard technology (SMR);
(5) Identify the most promising technologies for low carbon hydrogen production and the areas with greatest potential for cost effective emissions mitigation.
For the environmental assessments and for each production route, this study assesses both direct and indirect emissions associated with raw material extraction and conditioning, transportation of feedstock, processing and hydrogen production, as well as ancillary processes such as carbon capture and storage for each process route identified in Table 1. Indirect emissions include those associated with embodied emissions from infrastructure construction where available. The LCE data is calculated from figures quoted in the literature where all stages of production and use are specified. Whilst other environmental indicators (e.g. water usage, land-use changes, human health impacts) are important and may strengthen support for emerging technologies, only greenhouse gas (GHG) emissions are considered here. For the cost assessment, this study assesses both capital (Capex) and operating (Opex) costs associated with hydrogen production. The raw material extraction, conditioning, transportation and pre-processing is assumed to be included in the delivered price of raw materials unless otherwise directly specified.
The study considers hydrogen supplied at the facility gate, with no additional liquefaction or pressurization taken into account. Revised life cycle emissions estimates are based on updated supply chain emissions sources or calculations where specified. In the absence of transparent LCOH or LCE for a particular hydrogen production route, the full range of values available in the literature is reported and a sensitivity analysis across the range is performed. The levelized cost of carbon mitigation (eqn (1)) is expressed by a developed framework which compares the levelized cost of hydrogen ($ t−1 H2) and greenhouse gas life cycle emissions (LCE) (t CO2e t−1 H2) of individual hydrogen production technologies to the current industry standard technology, steam methane reforming (SMR).
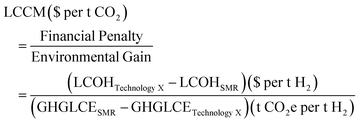 | (1) |
The LCCM is an estimate of the additional cost associated with reducing the emissions of hydrogen production per tonne of CO2e against emissions of hydrogen production using SMR. For fossil fuel routes, Carbon Capture and Sequestration (CCS) has been included. The reduction of CO2 emissions usually occurs via process efficiency improvements or direct substitution of existing technologies to less CO2-intensive alternatives. Marginal cost of abatement metrics, such as the carbon abatement cost (CAC) used for equipping CCS to SMR or coal gasification, are defined in relation to current technology. The LCCM metric compares the cost of decarbonization by technology substitution. Less-CO2 intensive technologies are typically more expensive than fossil technologies, resulting in a positive numerator for the LCCM metric. When a process is more expensive and more CO2 intensive from a life cycle perspective, the LCCM would be negative, meaning that technology substitution could not effectively reduce emissions. For example, the substitution of SMR for coal gasification without CCS results in higher emissions and cost, and is therefore not included in this analysis. All costs unless otherwise stated have been adjusted to 2016 USD using the Chemical Engineering Plant Cost Index (CEPCI).
3. Hydrogen costs and emissions inventories
The following section summarizes the costs and emissions inventories of each technology option presented in Table 1. For each route considered we present: a general process characterization, the current technology status, process costs and emissions, and key parameters governing process costs and emissions.3.1. Steam methane reforming
In conventional SMR, methane is reacted with steam using a catalyst at relatively high temperature, 650–1000 °C, and a pressure of 5–40 bar to produce carbon monoxide and hydrogen. Additional hydrogen is produced by reacting carbon monoxide with steam in the water–gas shift reaction.21 The overall process is represented by eqn (2)| CH4 + 2H2O ↔ CO2 + 4H2 ΔH0 = 165 kJ mol−1, ΔG0 = 114 kJ mol−1 | (2) |
The final stage of the process separates high-purity hydrogen (99.99%) from CO2 using pressure swing adsorption. Overall process efficiencies (CH4 HHV to H2 HHV) typically exceed 75%.22 SMR hydrogen costs are most sensitive to natural gas prices. Several correlations for hydrogen cost as a function of natural gas price are available. Gray and Tomlinson23 developed a relationship (eqn (3)) for large facilities with production capacities of approximately 100 million standard cubic feet per day (SCFD) (∼235 t H2 day−1) and capital costs of approximately $275–339 kg−1 H2 day−1. Capacities vary from 5000–250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mSTP3 h−1
000 mSTP3 h−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 in single and multi-train configurations. A similar relationship was presented by Penner25 without specifying plant size or operating assumptions (eqn (4)).
24 in single and multi-train configurations. A similar relationship was presented by Penner25 without specifying plant size or operating assumptions (eqn (4)).
| Hydrogen Cost (2001$ kg−1) = 0.137 × Natural Gas Price ($ GJ−1) + 0.1123 | (3) |
| Hydrogen Cost (2002$ kg−1) = 0.271 × Natural Gas Price ($ GJ−1) + 0.15 | (4) |
Using a natural gas price of $4 GJ−1, the HHV of hydrogen and adjusting to 2016 dollars, this corresponds to hydrogen costs of $0.91–$1.69 kg−1 H2 ($6.37–11.9 GJ−1). These cost estimates are in agreement with other studies predicting hydrogen costs of two to three times the cost of natural gas per unit of H2 produced.25 A summary of hydrogen production costs from SMR reported in the literature is shown in the ESI,† Table S1 and Fig. S1. The costs of current hydrogen production from these studies range from $1.03–1.57 kg−1 H2, with an average of $1.28 kg−1 H2 for natural gas prices from $3.25–8.31 GJ−1.
For literature studies including natural gas supply chain contributions to GHG emissions, the reported total range of LCE values are 10.72–15.86 kg CO2e kg−1 H2 (average of 12.4 of kg CO2e kg−1 H2)26–34 without CCS and 3.1–5.9 kg CO2e kg−1 H2 (average of 4.3 kg CO2e kg−1 H2) with CCS at 90% capture.27,28,32,33,35 Direct GHG emissions from the SMR hydrogen production phase are approximately 8–10 t CO2e t−1 H2, 60% of which is generated from the process chemistry, while the remaining 40% arises from heat and power sources required.36 The majority of CO2 produced exits in two streams, a diluted stream (stack gases with CO2 concentration 5–10% vol) and a concentrated stream (approximately 50% by vol after pressure swing adsorption).37 The relatively pure CO2 stream is readily amenable for lower-cost CCS if appropriate geological storage reservoirs are located nearby. If deep decarbonisation is required and emissions must be further reduced from the entire process, then an amine solvent (MEA) based CCS process might be used to capture up to 90% of the CO2 contained in the stack gases,38 although demonstrated removal rates are typically 80%.39 LCE emissions estimates are most sensitive to direct process emissions and fugitive emissions associated with the natural gas supply chain.
Methane emissions, which occur from vents, incomplete combustion and fugitive leaks, exhibit high variability and are heavily skewed in distribution: a small number of facilities exhibit large emissions.87 The contribution of fugitive methane emissions to overall LCE is exacerbated by the high relative climate forcing of methane compared to CO2, especially over short timescales.
For this study, a central estimate (median) of 0.9% of total gas delivered is used, with a range of 0.6–1.4%,‡ taken from Balcombe et al.87 as this reflects interquartile range of emissions associated lower-emitting supply chains utilising modern equipment and effective operation. Given that the context of this paper is regarding new hydrogen production opportunities to lower emissions, the authors argue that this is an appropriate assumption. Note that this includes the whole supply chain including low pressure distribution, which is not likely to be part of the hydrogen supply chain. Thus, these may be a small overestimate of emissions. These emissions values equate to 1.1 kg CO2e kg−1 H2 with a range of 0.8–1.9 kg CO2e kg−1 H2 based on a global warming 100 year time horizon value of 36 g CO2e g−1 CH488 and overall 76% HHV process efficiency.
CO2 emissions associated with the natural gas supply chain are typically due to fuel usage for processing and transport and are estimated to have a median of 10 g CO2 MJ−1 HHV delivered gas with an interquartile range of 8.2–14.8 g CO2 MJ−1 HHV for best practice supply chains.87 The variation in emissions primarily arises from different raw gas compositions and transport distances. The raw gas composition governs the fuel required for processing: higher fraction of impurity requires more fuel for processing. Additionally, raw gas with high CO2 content would vent more CO2 once separated. Compressor stations are required at set distances to boost pressure and thus a longer distance requires more fuel for compressor duty.
For unabated SMR, using the updated supply chain emissions contributions, this is equivalent to 2.95 kg CO2 kg−1 H2 for the mean case, with a range of 2.25–4.47 kg CO2 kg−1 H2 using an overall 76% HHV process efficiency. Thus, this study estimates a mean total LCE value of 12.83 kg CO2e kg−1 H2, with a range of 10.1–17.21 kg CO2e kg−1 H2. For SMR with CCS (90% capture), the supply chain contributions total 3.3 kg CO2e kg−1 H2 for the median case, with a range of 2.52–5.0 kg CO2e kg−1 H2 using an overall 68% HHV process efficiency. The total LCE estimate for SMR with CCS (90% capture) is 5.61 kg CO2e kg−1 H2 with a range of 2.97–9.16 kg CO2e kg−1 H2. The LCE values estimated here represent updated natural gas supply chain ranges combined with the ranges of direct process emissions presented in the literature, including external electricity contributions of ∼0.7 kg CO2e kg−1 H2.33,35,36 Combining low-range supply chain estimates with low-range point-source creates realistic lower bound estimates (and vice versa for upper limits) for overall process emissions.
3.2. Coal gasification
In conventional coal gasification, pulverized coal is partially oxidized with air or oxygen at high temperatures (800–1300 °C) and pressures of 30–70 bar, producing a syngas mixture composed of H2, CO, CO2 and CH4 (and other impurities, e.g. COS, H2S).40 The raw syngas undergoes a water–gas shift reaction to increase the hydrogen yield. The syngas is scrubbed to remove particulates, sulphur containing compounds and approximately 50% of the carbon dioxide prior to hydrogen separation using PSA.23 Waste gases from the PSA, rich in CO2 but also some H2 and CO, can be used for power generation to offset the high energy requirements of plant or for export electricity as a co-product to the hydrogen generated.24 The overall reaction stoichiometry can be represented by eqn (5).| CH0.8 + 0.6O2 + 0.7H2O → CO2+ H2 ΔH0 = 90 kJ mol−1, ΔG0 = 63 kJ mol−1 | (5) |
Compared to SMR, coal gasification plants are less efficient (∼55% fuel-to-hydrogen HHV) more complex and have larger single train capacities, typically ranging from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 100
000 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mSTP3 h−1.24 Hydrogen costs from coal gasification are most sensitive to facility capital costs. It has been shown previously that if the coal price changed by 25 percent, the hydrogen costs only increase by approximately $0.05 kg−1.22 The relationship between total capital cost and plant capacity presented by Konda et al.41 is shown in eqn (6). Coal gasification is a commercially mature technology, but due to the higher capital costs, lower efficiencies and increased complexity for solid-fuel gasification techniques, hydrogen production costs are typically only competitive with SMR where oil and/or natural gas is expensive compared to coal.42 Estimates of the cost of hydrogen available in the literature are $0.96–1.88 kg H2 (average of $1.38 kg−1 H2) for coal feedstock prices from $1.33–2.73 GJ−1 (summarised in ESI,† Table S3).
000 mSTP3 h−1.24 Hydrogen costs from coal gasification are most sensitive to facility capital costs. It has been shown previously that if the coal price changed by 25 percent, the hydrogen costs only increase by approximately $0.05 kg−1.22 The relationship between total capital cost and plant capacity presented by Konda et al.41 is shown in eqn (6). Coal gasification is a commercially mature technology, but due to the higher capital costs, lower efficiencies and increased complexity for solid-fuel gasification techniques, hydrogen production costs are typically only competitive with SMR where oil and/or natural gas is expensive compared to coal.42 Estimates of the cost of hydrogen available in the literature are $0.96–1.88 kg H2 (average of $1.38 kg−1 H2) for coal feedstock prices from $1.33–2.73 GJ−1 (summarised in ESI,† Table S3).
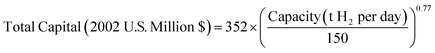 | (6) |
The lower C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H ratio in coal relative to natural gas results in significantly higher direct CO2e emissions from the process (14.4–25.31 kg CO2e kg−1 H2), with an average value of 19.14 kg CO2e kg−1 H2.8,22,28,43–45 The total LCE of unabated coal-to-hydrogen is most sensitive to the direct hydrogen conversion steps.
H ratio in coal relative to natural gas results in significantly higher direct CO2e emissions from the process (14.4–25.31 kg CO2e kg−1 H2), with an average value of 19.14 kg CO2e kg−1 H2.8,22,28,43–45 The total LCE of unabated coal-to-hydrogen is most sensitive to the direct hydrogen conversion steps.
Underground coal mining emissions factors vary with basin-specific coal conditions and depth of the coal extracted.90–92 In the absence of mine specific data, IPCC guidelines estimate Tier 1 emissions factors for underground coal mining without coal mine methane mitigation range from 10–25 m3 CH4 t−1 coal, which introduces considerable uncertainty. This corresponds to a range of 2.06–5.15 kg CO2e kg−1 H2 using a HHV of 30.2 MJ kg−1, an overall coal-to-hydrogen efficiency of 55% and a methane GWP of 36. Accounting for coal processing and transportation, the total upstream emissions value for underground coal mining ranges from 2.51–5.6 kg CO2e kg−1 H2, with an average of 4.5 kg CO2e kg−1 H2.
This study estimates total LCE values of 2.11 kg CO2e kg−1 H2 (range 1.09–5.52 kg CO2e kg−1 H2) and 19.78 kg CO2e kg−1 H2 (range 14.72–26.09 kg CO2e kg−1 H2) for surface mined coal with (90% capture) and without CCS respectively. For underground mined coal, this study estimates 23.85 kg CO2e kg−1 H2 and 6.2 kg CO2e kg−1 H2 with ranges of 16.9–30.9 kg CO2e kg−1 H2 and 2.76–9.57 kg CO2e kg−1 H2 for underground mined coal with and without CCS (90% capture) respectively. The LCE values estimated here represent updated supply chain contributions to the range of LCE values in the literature. For the levelized cost of mitigation and decarbonization fraction calculations (see Section 4.1), this study uses an LCE for coal with CCS of 4.6 kg CO2e kg−1 H2 (average across surface and underground mine estimates), with a sensitivity of the full range of supply chain emissions ranges.
The most recent study reporting a CAC for hydrogen from coal gasification was conducted in 2007. Given the CAC trend from the SMR time series over the same time period (Fig. 1) and the substantially lower CAC values reported by coal-to-hydrogen studies (Table S4, ESI,† reflective of the SMR costs in the early 2000's), the cost of equipping CCS for coal gasification to hydrogen routes was sourced from more recent detailed coal-to-power studies with CCS. For power production from coal with pre-combustion capture (necessary for H2 generation), integrated gasification combined cycle (IGCC) plants provide the most economical route. However, for many recent studies100–102 the CAC reference plant for IGCC facilities for power production is a supercritical pulverized coal (SCPC) plant without capture (not a similar IGCC plant without capture), which would be the lower cost route for coal-fired power plants without capture.45 This results in an increase in the average CAC from $43.92 t−1 CO2 (for IGCC with CCS to IGCC without) to $77.34 t−1 CO2 (excluding transport and storage for both) as reported in a recent review by Rubin et al.45 An average value of $43.92 t−1 CO2 for IGCC compared to a SMR baseline has been used.45 Including the ZEP transport and storage costs of $22 t−1 CO2, the total CCS cost for 90% capture from coal gasification used in this study is $65.92 t−1 CO2.
It is noteworthy the CAC value used here is significantly higher than that for coal with CCS as shown by the literature studies in Table S4 (ESI†) ($17.26–25.19 t−1 CO2 for capture and compression and $6.85–15.34 t−1 CO2 for transport and storage). However, it is in agreement with the average cost of hydrogen produced from coal with ($2.17 kg−1 H2) and without CCS ($1.40 kg−1 H2), and the average life cycle emissions reported in the literature for each (18.37 kg CO2 kg−1 H2 and 2.6 kg CO2 kg−1 H2 for 90% capture, respectively). This results in a CAC of $58 t−1 CO2.
3.3. Biomass gasification
Hydrogen can be produced from biomass resources such as wood, agricultural residues, consumer wastes or crops grown specifically for energy purposes.43 For hydrogen generation, biomass processing pathways include gasification, pyrolysis, supercritical extraction, liquefaction and hydrolysis. Gasification has the highest hydrogen yield per unit feedstock and is the focus here. Biomass gasification is closely related to coal gasification, consisting of steam gasification, gas cleaning (ash and particular removal), water–gas shift and hydrogen separation via pressure swing adsorption.24 In general, biomass does not gasify as easily as coal and produces other hydrocarbons exiting the gasifier. Biomass gasification occurs at temperatures from 500–1400 °C and operating pressures from atmospheric to 33 bar depending on plant scale and reactor configuration.49 The general reaction is shown in eqn (7).| Biomass + H2O → H2 + CO + CO2 + CH4 + Tar + Char | (7) |
Despite the development of several system components and designs, the process is still largely uncommercialized for hydrogen production, with some promising pilot scale facilities for bio-methane and bio-hydrogen underway.50,51 Like coal gasification, hydrogen production costs from biomass are most sensitive to the high cost of capital. Large-scale facilities are required to offset the high capital costs of a complex solid fuels gasification facility.52 Overall biomass-to-hydrogen efficiencies (HHV) for large scale facilities of 52% have been estimated.53 Hydrogen production costs are estimated to be $1.82–2.11 kg−1 for 139.7 t H2 day−1 output for biomass costs of $47.4–82.5 dry ton−1.54 Removing studies concerning future biomass hydrogen production costs and the outliers for very small production facilities reported by Yao et al.,55 the LCOH from biomass sources in the literature ranges from $1.48–3.00 kg−1 H2, with a mean value of $2.24 kg−1 H2 for biomass feedstock prices of $48.37–115.2 t−1 dry biomass delivered (summarised in Table S5, ESI†).
Although not currently economically competitive, biomass gasification is an attractive option for recovering energy from domestic and agricultural waste. Since CO2 is fixed by photosynthesis from growing processes, it is one of only a few options that may result in net negative CO2 emissions when implemented with CCS.56 Only one study concerning the LCOH from biomass gasification coupled with CCS was found,22 which reported a value a $2.27 kg−1 H2.
Several LCA studies have been conducted in the field of hydrogen production via biomass gasification. The LCE values reported range from 0.31–8.63 kg CO2e kg−1 H2,57–62 with an average of 2.59 kg CO2e kg−1 H2. The large range stems from variations in biomass feedstocks (e.g. waste or different types of energy crop) and transportation requirements. The inclusion of land-use change effects may also add to environmental impacts significantly, which is not considered here and not well studied in active literature. Only one study investigating biomass gasification with CCS for hydrogen production was conducted by Susmozas et al.63 This study reported the gasification of poplar biomass with capture and sequestration of 70% of the produced CO2, resulting in an overall LCE of −14.58 kg CO2 kg−1 H2. Due to the similarities between coal gasification and biomass gasification pathways, the CAC of $65.92 t−1 CO2 for coal gasification is used as the avoidance cost for equipping CCS to biomass gasification. It should be noted that due to commercial immaturity and required reactor modifications, this is likely an underestimate of the cost of CCS with biomass gasification.
3.4. Methane pyrolysis
Pyrolysis involves the decomposition of hydrocarbons at high temperatures (thermally or catalytically) in non-oxidative environments to produce hydrogen and solid carbon (eqn (8)). Methane represents the most promising hydrocarbon for pyrolysis given its hydrogen-to-carbon ratio and large reserves (in particular if hydrates are included64). Since no water or air is present during reaction, no carbon oxides (CO or CO2) are formed, eliminating the need for secondary reactors (i.e. water–gas shift).8| CH4 ↔ C + 2H2 ΔH0 = 75.6 kJ mol−1, ΔG0 = 63 kJ mol−1 | (8) |
Without the inclusion of the water–gas shift and preferential oxidation to CO reactions, processing for CH4 decomposition is greatly simplified relative to SMR.65 Additionally, the higher H2 concentration of the gas stream exiting the reactor for methane pyrolysis has the potential for a considerable reduction in capital and operating costs due to less downstream processing required to produce commercial-grade H2 relative to SMR.65 The thermal decomposition of natural gas has been extensively investigated for the formation of valuable carbon products and is commercially practiced to produce carbon black for use in tires and electrical equipment.66 Approximately 95% of the global carbon black market demand (∼16.4 million tonnes) is supplied by non-catalytic methane pyrolysis. From the stoichiometry of eqn (8), if the global hydrogen demand were supplied by pyrolysis the carbon produced would be approximately 12 times the size of the carbon black market. For hydrogen production the technology is less mature with only a handful of pilot to early-stage commercial sites in operation.66
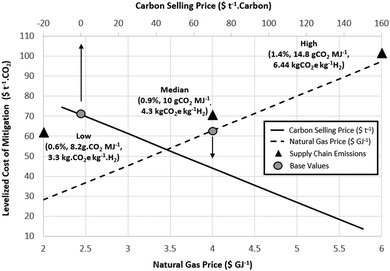
The economics of methane pyrolysis are strongly determined by the natural gas price and value of the carbon by-product. The available literature studies concerning LCOH range from $1.03–2.45 kg−1 H2, with an average of $1.75 kg−1 H2 for natural gas prices from $3.2–6.8 GJ−1 (summarised in Table S8, ESI†). Literature estimates of the LCOH alongside assumed natural gas price and carbon product value are shown in Fig. 2. Depending on the catalyst used, the carbon product formed can provide an additional revenue stream. Carbon values in the literature studies range from $0–300 t−1, whereas potential carbon product values range from $0–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t−1 for valuable carbon modifications (graphite, carbon fibers etc.).66 The preferred temperature ranges, carbon products and catalyst selections have been studied in detail elsewhere and are not discussed here.9,67 Large-scale deployment of pyrolysis technologies for decarbonisation would quickly surpass demand for carbon in the absence of new market developments. For a conservative approach in this study, a LCOH of $1.76 kg−1 H2 is estimated based on a recent model developed by Parkinson et al.,71 using a natural gas price of $4 GJ−1 and a carbon product value of zero. The range of cost estimates presented in Table 5 are generated by varying the carbon product value from $−10 to 150 t−1 (negative value reflects a no value product with a small disposal cost).
000 t−1 for valuable carbon modifications (graphite, carbon fibers etc.).66 The preferred temperature ranges, carbon products and catalyst selections have been studied in detail elsewhere and are not discussed here.9,67 Large-scale deployment of pyrolysis technologies for decarbonisation would quickly surpass demand for carbon in the absence of new market developments. For a conservative approach in this study, a LCOH of $1.76 kg−1 H2 is estimated based on a recent model developed by Parkinson et al.,71 using a natural gas price of $4 GJ−1 and a carbon product value of zero. The range of cost estimates presented in Table 5 are generated by varying the carbon product value from $−10 to 150 t−1 (negative value reflects a no value product with a small disposal cost).
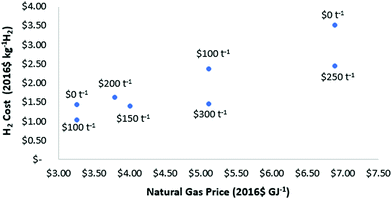 | ||
| Fig. 2 Summary of the LCOH from pyrolysis processes available in the literature.32,48,69,71,103–105 Labelled values represent the carbon sale price ($ t−1 carbon) assumed in the study. | ||
The GHG emissions values of methane pyrolysis processes are heavily dependent on the fuel source for heating and whether the study included emissions contributions from the natural gas supply chain. The direct emissions could potentially be as low as 1.1 kg CO2 kg−1 H2 given the process chemistry if methane was used as the process fuel, or even zero if 30–35% of the hydrogen product was burned. Several studies35,68–70 report the direct emissions of a pyrolysis facility to range from 0.2–2.5 kg CO2 kg−1 H2. Rudimentary LCAs including upstream fugitive emissions estimate LCE of 1.9–5.5 kg CO2 kg−1 H2.14,21,71 The highest value of 5.5 kg CO2 kg−1 H2 reported by Machhammer et al.14 utilized an electric heater as a heat source resulting in higher emissions per kg of hydrogen product.
For methane pyrolysis using the updated supply chain emissions discussed in Section 3.1, this study estimates the supply chain emissions contribute 4.28 kg CO2e kg−1 H2 for the median case, with a range of 3.26–6.44 kg CO2e kg−1 H2 using an overall process efficiency of 53% HHV.71 This study estimates, using natural gas as a heat source, a mean LCE of 6.1 kg CO2e kg−1 H2, with a range of 4.2–9.14 kg CO2e kg−1 H2. This is higher than any of the LCE values reported in the literature due to the previously underestimated or not included supply chain contribution to overall LCE of a pyrolysis facility. Compared to SMR, more natural gas is required per unit hydrogen product, which increases the importance of accurate supply chain contributions to the overall life cycle emissions.
3.5. Electrolysis routes
The electrolysis of water to hydrogen (eqn (9)) is a commercially mature process. It is a promising technology for storing surplus energy from intermittent renewable sources in the form of hydrogen.72 Low-carbon electrolytic hydrogen requires electricity from low-carbon technologies such as solar PV, wind turbines or nuclear power. The developed and commonly used electrolyzer technologies are alkaline, proton exchange membrane (PEM) and solid-oxide electrolysis cells (SOEC).72 Alkaline water electrolysis using an aqueous potassium hydroxide solution is the most commercially mature of the technologies, providing the lowest capital costs but is limited by low current densities (0.2–0.5 A cm−2) resulting in high electrical energy costs.52 SOEC are the most electrically efficient of the three technologies, but are currently in the R&D stage facing challenges with corrosion, seals, thermal cycling and chromium migration.73 PEM electrolysis is currently more expensive than alkaline technologies, but is an attractive future technology due to higher current densities (>2 A cm−2), higher efficiencies (the largest cost driver), dynamic operation and compact system design.74 An excellent summary of the technical specifications of each technology has been compiled by Bhandari et al.28 | (9) |
Estimates of LCE of electrolytic hydrogen vary widely across the literature and the use of primary data has been very limited. For wind electrolysis, a detailed report prepared at NREL93 is the major data source (0.97 kg CO2e kg−1 H2) for every paper discussing LCA of this method. Additionally, none of the studies considered the emissions contribution of the electrolyzer unit. The same applies for nuclear based electrolytic processes, of which only two independent primary sources94,95 were found. For solar PV based electrolysis, the values vary by over a factor of two. Little data is available on the LCE contribution from electrolyser manufacturing and replacements, with estimates suggesting a relatively small contribution in the range of 30–50 g CO2e kg−1 H228,76,77 depending on operating lifetime utilization rates. The available LCE estimates from the literature for wind electrolysis range from 0.8–2.2 kg CO2e kg−1 H2 (average of 1.34 kg CO2e kg−1 H2), 1.99–7.1 kg CO2e kg−1 H2 (average of 4.47 kg CO2e kg−1 H2) for solar electrolysis and 0.47–2.13 kg CO2e kg−1 H2 (average of 1.65 kg CO2e kg−1 H2) for nuclear electrolysis.
LCE values are re-estimated in this study (Table 3), calculated from the contributions of the LCE per kW h of the energy generation technology and the manufacture of the electrolyzer (Fig. 3) using an overall energy requirement of 51.2 kW h kg−1 H2. The overall energy requirement was calculated from an optimistic 85% cell voltage efficiency with a total balance of plant load 5.04 kW h kg−1 H2.75 For wind and solar electrolysis routes, emissions estimates were taken from a review of 153 life cycle studies of wind and solar power generation by Nugent et al.96 The interquartile ranges of LCE per kW h are 9.4–21.4 g CO2e kW h−1 (median of 16.6 of CO2e kW h−1) and 25–48 g CO2e kW h−1 (median of 42.4 g CO2e kW h−1) for wind and solar power respectively. This generates ranges of 0.52–1.14 kg CO2 kg−1 H2 (central estimate 0.88 kg CO2 kg−1 H2) and 1.32–2.5 kg CO2 kg−1 H2 (central estimate 2.21 kg CO2 kg−1 H2) for wind and solar electrolysis respectively.
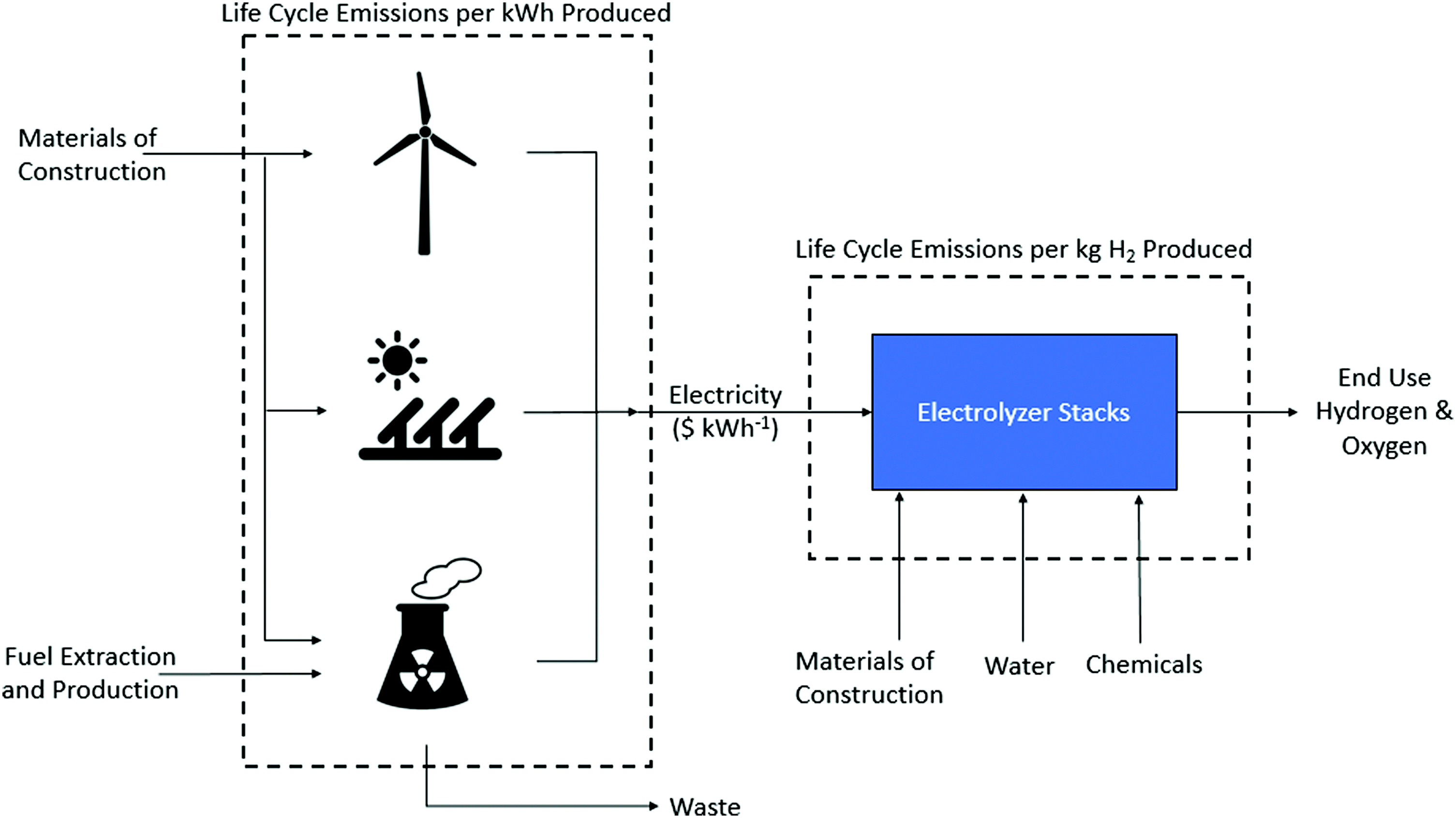 | ||
| Fig. 3 Renewable electrolysis routes considered. LCE for energy generation technologies and electrolysers considered independently on a kg CO2e kW h−1 and kg CO2e kg−1 H2 basis respectively. | ||
Several of the nuclear electrolysis routes shown in Table S7 (ESI†) are high-temperature electrolysis processes using SOFC. Here PEM electrolysis with nuclear power as a feedstock is used. The LCE per kW h generated for nuclear power were taken from a systematic review of 99 independent estimates conducted by Warner et al.,97 with an interquartile range of 8.4–18 g CO2e kg−1 H2 (median of 14 g CO2e kg−1 H2). The generates total LCE estimates of 0.47–0.96 kg CO2 kg−1 H2, with a central estimate of 0.76 kg CO2 kg−1 H2 for nuclear electrolysis. Interquartile ranges were used due to the relatively small data sets skewing standard deviations and 5th–95th percentile ranges. As emissions intensity per kW h varies based on utilization factors, a sensitivity analysis of the LCE of each technology was performed using the interquartile ranges of LCE for each technology (Section 4.4).
 | (10) |
The available literature studies concerning LCOH from wind electrolysis range from $3.56–9.08 kg−1 H2 (average of $5.64 kg−1 H2), $3.34–17.30 kg−1 H2 (average of $10.89 kg−1 H2) for solar electrolysis and $1.95–6.20 kg−1 H2 (average of $4.24 kg−1 H2) for nuclear electrolysis. Electrolyzer capital costs vary significantly across studies, from $300–1300 kW−1 with different resource availability estimates, assumed energy generation capital or LCOE supply. A summary of the LCOH of the various routes from literature is shown in the ESI,† Table S7. Hydro-power based electrolysis has been excluded from the analysis due to limited resource availability given the geographic requirements of the technology. However, as the cost of hydrogen produced by electrolysis can be described by a linear function of the levelized cost of electricity, it can be determined for specific locations and embodied emissions per kW h.
In this study the LCOH was estimated with a discounted cash flow analysis using the LCOE from each energy generation technology and standard capacity factors (Table 2). PEM was chosen as the reference electrolyser technology operating with an 85% cell voltage efficiency (∼51 kW h kg−1 H2).106 For wind and solar technology routes, the lower LCOE sensitivity bounds are the projected minimum LCOE costs based on technology learning curves to 2050 projected by Wiser et al.107 and the IEA108 respectively. The lower bound LCOE for nuclear power is based on mid-range future estimates by the World Nuclear Association,109 although they note drawing any strong conclusions about future nuclear power costs based on a specific countries experience may not be indicative of future pricing. This generates cost ranges of $4.61–10.01 kg−1 H2 (central estimate $7.86 kg−1 H2), $7.1–14.87 kg−1 H2 (central estimate of $12.00 kg−1 H2) and $4.99–8.21 kg−1 H2 (central estimate of $6.79 kg−1 H2) for wind, solar and nuclear electrolysis respectively. Further assessment assumptions are detailed in the ESI,† Tables S9–S12.
| Technology | Average life cycle CO2 emissions per kW h produced (g CO2e kW h−1) | On-stream factor (%) | LCOE ($ kW h−1) | Electrolyzer CAPEX range72 ($ kW−1) |
|---|---|---|---|---|
| Wind electrolysis | 16.6 (9.4–21.4) | 33 | 0.06 (0.04–0.08) | 800 (400–1000) |
| Solar PV electrolysis | 42.4 (25–48) | 20 | 0.08 (0.056–0.1) | |
| Nuclear electrolysis | 14 (8.4–18) | 95 | 0.1 (0.08–0.12) |
3.6. Nuclear thermochemical cycles
The inefficiencies of several energy conversion steps from nuclear heat to hydrogen via electrolysis has prompted significant research into direct use of the nuclear heat for water splitting via thermochemical cycles.54 Over 100 different thermochemical cycles have been identified,78 but only twenty five cycles were found to be feasible by Brown et al.79 At present, the most promising high-temperature and low-temperature cycles are the sulphur–iodine (S–I) cycle and copper–chloride (Cu–Cl) cycle, respectively.80 The S–I cycle is a thermally driven cycle, described by eqn (11)–(14).13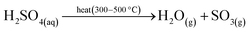 | (11) |
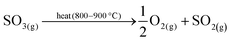 | (12) |
| SO2(g) + I2(g) + 2H2O(l) → 2HI(g) + H2SO4(aq) | (13) |
 | (14) |
As there are no side reactions from eqn (10)–(13), recycling the products of each step is a relatively straightforward process.81 The reliance on high temperature to drive the reactions requires either nuclear power or concentrated solar, of which nuclear power has been the primary research focus due to intermittency of solar resources. The Cu–Cl cycle is less efficient than the S–I cycle (∼40% vs. 60%, respectively), but operates at lower temperatures (350–550 °C) significantly reducing the engineering challenges.80 The most common Cu–Cl cycle is characterized by five main steps shown in eqn (15)–(19).
 | (15) |
 | (16) |
 | (17) |
 | (18) |
 | (19) |
Available studies concerning the cost of hydrogen produced by the above thermochemical water splitting cycles are shown in the ESI,† Table S8. The LCOH for the S–I and Cu–Cl cycles range from $1.47–2.71 kg−1 H2 and $1.47–2.7 kg−1 H2 with averages of $1.81 kg−1 H2 and 2.13 kg−1 H2 respectively. Thermochemical cycle cost data is limited, with none of the studies publishing the capital cost data used to determine the LCOH. Thus, there is significant and unquantifiable uncertainty in the LCOH estimates. Deployment of the thermochemical cycles have so far been limited to pilot-scale testing by the Japan Atomic Energy Agency and further R&D by the Idaho National Laboratory. Large production scales would be required to match the heat produced by nuclear facilities built at-scale.
The LCE values reported for the S–I and Cu–Cl cycles range from 0.41–2.2 kg CO2 kg−1 H212,13,82 and 0.7–1.8 kg CO2 kg−1 H2,12,13,83 with averages of 1.2 kg CO2 kg−1 H2, 1.08 kg CO2 kg−1 H2 respectively. As no greenhouse gas emissions are produced from the operation of thermochemical water cycles, the life cycle emissions are generated from the materials of construction, replacement of inventory and waste disposal. There is significant uncertainty in the limited emissions estimates available as the sources of contributions to overall emissions values are not transparent.
3.7. Summary of hydrogen costs and emissions inventory
A summary of the ranges in the literature of emissions for each technology route is compared to the re-estimates in this study is given in Table 3. In the absence of detailed quantitative data for the supply chain analyses for biomass gasification and thermochemical nuclear cycles, no emissions re-estimates from existing literature have been made. More robust supply chain analysis is required for these technologies for appropriate ranges to be deduced and should be the subject of future research. Likewise, a summary of the literature cost data versus re-estimates in this study is given in Table 4. No updated estimates are provided for unabated SMR and coal gasification as they are technologically mature with well-established cost structures. No updated estimates are provided for biomass gasification and nuclear thermochemical cycles due to the absence of detailed and transparent quantitative data.4. Results
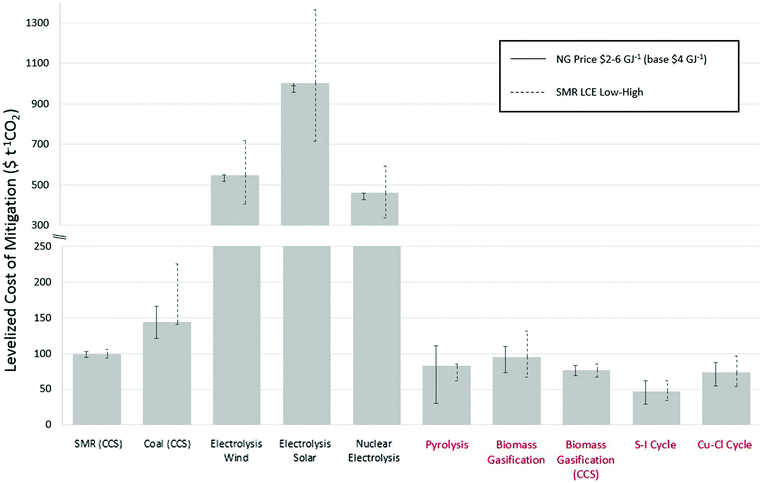
4.1. The levelized cost of carbon mitigation
The LCCM was calculated using eqn (1) using the re-estimated central LCOH and LCE values from Section 3. In the absence of updated values for some technology routes, the median reference values from the literature presented in Tables 3 and 4 are used to determine the LCCM. The estimates of the LCCM are given in Fig. 4 for each technology relative to the baseline SMR values.| Technology | Literature estimates (kg CO2e kg−1 H2) | Our estimates (kg CO2e kg−1 H2) | ||||
|---|---|---|---|---|---|---|
| Low | Central | High | Low | Central | High | |
Our “Low–Central–High” estimates utilize a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) supply chain contributions of 0.6–1.4% (central 0.9%) fugitive methane emissions and 8.2–14.8 g CO2 MJ−1 HHV (central 10 g CO2 MJ−1 HHV) to the full emissions range presented in the literature, b supply chain contributions of 0.6–1.4% (central 0.9%) fugitive methane emissions and 8.2–14.8 g CO2 MJ−1 HHV (central 10 g CO2 MJ−1 HHV) to the full emissions range presented in the literature, b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) the IPCC Tier 1 emissions ranges of 10–25 m3 CH4 t−1 for underground coal and 0.32–0.77 kg CO2e kg−1 H2 (central estimate of 0.45 kg CO2e kg−1 H2) for surface mined coal supply chain contributions to the full emissions range presented in the literature, c the IPCC Tier 1 emissions ranges of 10–25 m3 CH4 t−1 for underground coal and 0.32–0.77 kg CO2e kg−1 H2 (central estimate of 0.45 kg CO2e kg−1 H2) for surface mined coal supply chain contributions to the full emissions range presented in the literature, c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±20% of the single reference study, d ±20% of the single reference study, d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) the interquartile ranges of the g kW h−1 emissions from power generation study reviews (Section 3.5) combined with electrolyser contributions of 40 g CO2e kg−1 H2. e the interquartile ranges of the g kW h−1 emissions from power generation study reviews (Section 3.5) combined with electrolyser contributions of 40 g CO2e kg−1 H2. e![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) First value represents total LCE estimates from surface mined coal and the second value total LCE estimates from underground mined coal. First value represents total LCE estimates from surface mined coal and the second value total LCE estimates from underground mined coal. |
||||||
| SMRa | 10.72 | 12.4 | 15.86 | 10.09 | 13.24 | 17.21 |
| SMR w. CCSa | 3.1 | 4.3 | 5.92 | 2.97 | 5.61 | 9.16 |
| Coalb | 14.4 | 19.14 | 25.31 | 14.72/16.9e | 19.78/23.85e | 26.09/30.9e |
| Coal w. CCSb | 0.78 | 1.8 | 5.2 | 1.09/3.27e | 2.11/6.2e | 5.52/10.35e |
| CH4 pyrolysisa | 1.9 | 3.72 | 5.54 | 4.2 | 6.1 | 9.14 |
| Biomass | 0.31 | 2.6 | 8.63 | 0.31 | 2.6 | 8.63 |
| Biomass w. CCSc | −14.58 | −11.66 | −14.58 | −17.50 | ||
| Electrolysis windd | 0.85 | 1.34 | 2.2 | 0.52 | 0.88 | 1.14 |
| Electrolysis solard | 1.99 | 4.47 | 7.1 | 1.32 | 2.21 | 2.5 |
| Electrolysis nucleard | 0.47 | 1.65 | 2.13 | 0.47 | 0.76 | 0.96 |
| S–I cycle | 0.41 | 1.2 | 2.2 | 0.41 | 1.2 | 2.2 |
| Cu–Cl cycle | 0.7 | 1.08 | 1.8 | 0.7 | 1.08 | 1.8 |
| Technology | Literature estimates ($ kg−1 H2) | Our estimates ($ kg−1 H2) | ||||
|---|---|---|---|---|---|---|
| Low | Central | High | Low | Central | High | |
Our “Low–Central–High” estimates use a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) an updated SMR CCS cost of $96.15 t−1 CO2 ±20% for a 90% point source capture scenario from the literature median hydrogen production cost of $1.26 kg−1 H2, b an updated SMR CCS cost of $96.15 t−1 CO2 ±20% for a 90% point source capture scenario from the literature median hydrogen production cost of $1.26 kg−1 H2, b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) an updated coal gasification CCS cost of $65.92 t−1 CO2 ±20% for a 90% point source capture scenario from the literature average cost of $1.38 kg−1 H2, c an updated coal gasification CCS cost of $65.92 t−1 CO2 ±20% for a 90% point source capture scenario from the literature average cost of $1.38 kg−1 H2, c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) adjusted carbon sale price from $−10 to 150 t−1 carbon product for $4 GJ−1 natural gas cost, d adjusted carbon sale price from $−10 to 150 t−1 carbon product for $4 GJ−1 natural gas cost, d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) an updated biomass gasification CCS cost of $65.92 t−1 CO2 ±20% for a 90% point source capture scenario from the literature reference cost of $2.27 kg−1 H2, and e an updated biomass gasification CCS cost of $65.92 t−1 CO2 ±20% for a 90% point source capture scenario from the literature reference cost of $2.27 kg−1 H2, and e![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) the technology specific LCOE and capital cost bounds shown in Table 2 and economic assumptions shown in Tables S9–S12 (ESI). the technology specific LCOE and capital cost bounds shown in Table 2 and economic assumptions shown in Tables S9–S12 (ESI). |
||||||
| SMR | 1.03 | 1.26 | 2.16 | 1.03 | 1.26 | 2.16 |
| SMR w. CCSa | 1.22 | 1.88 | 2.81 | 1.93 | 2.09 | 2.26 |
| Coal | 0.96 | 1.38 | 1.88 | 0.96 | 1.38 | 1.88 |
| Coal w. CCSb | 1.4 | 2.17 | 3.6 | 2.24 | 2.46 | 2.68 |
| CH4 pyrolysisc | 1.03 | 1.75 | 2.45 | 1.36 | 1.76 | 1.79 |
| Biomass | 1.48 | 2.24 | 3.00 | 1.48 | 2.24 | 3.00 |
| Biomass w. CCSd | — | 2.27 | — | 3.15 | 3.37 | 3.6 |
| Electrolysis winde | 3.56 | 5.24 | 10.82 | 4.61 | 7.86 | 10.01 |
| Electrolysis solare | 3.34 | 8.87 | 17.3 | 7.1 | 12.00 | 14.87 |
| Electrolysis nucleare | 3.29 | 4.63 | 6.01 | 4.99 | 6.79 | 8.21 |
| S–I cycle | 1.47 | 1.81 | 2.71 | 1.47 | 1.81 | 2.71 |
| Cu–Cl cycle | 1.47 | 2.13 | 2.7 | 1.47 | 2.13 | 2.7 |
As the LCCM value for each technology is dependent on its own LCOH and LCE, as well as the reference values used for SMR, the error bars in Fig. 4 show the sensitivity of varying the SMR reference values by changing the natural gas price and overall LCE. The natural gas price has been varied from $2–6 GJ−1 to reflect variation in region-specific prices and future uncertainty, and the overall SMR LCE from low to high (10.09–17.21 kg CO2e kg−1 H2). In addition to changing the reference value, the varying natural gas prices and supply chain contributions to LCE also directly affects natural gas using technologies such as SMR with CCS and pyrolysis.
4.2. Variation of key parameters affecting costs and emissions inventories
In this section we determine the impact of varying the key underlying parameters influencing the levelized cost and life cycle emissions for each hydrogen production technologies on the LCCM. The parameter ranges used for this sensitivity analysis are presented in Table 5 by hydrogen production technology. The results of this sensitivity analysis are presented in Fig. 5 and the most promising technologies and areas for innovation are discussed in the remainder of this section.| Technology | LCOH sensitivity parameters | LCE sensitivity parameters |
|---|---|---|
| SMR + CCS | CCS CAC price (±20% of $96 t−1 CO2) | Full LCE range 90% capture (2.97–9.16 kg CO2 kg−1 H2) |
| Coal gasification + CCS | CCS CAC price (±20% of $65.92 t−1 CO2) | Full LCE range 90% capture (1.09–10.35 kg CO2 kg−1 H2) |
| Methane pyrolysis | Carbon selling price (−$10–150 t−1 carbon) | Full LCE range (4.2–9.14 kg CO2 kg−1 H2) |
| Biomass gasification | LCOH (literature range 1.48–3.00 kg−1 H2) | LCE literature range (0.41–8.63 kg CO2 kg−1 H2) |
| Biomass gasification + CCS | CCS CAC price (±20% of $65.92 t−1 CO2) | LCE literature (±20% of LCE value of −14.58 kg CO2 kg−1 H2) |
| Electrolysis – wind | LCOE & electrolyzer capex ($0.04–0.08 kW h−1, $400–1000 kW−1) | Wind power carbon intensity (9.4–21.4 g CO2 kW h−1) |
| Electrolysis – solar | LCOE & electrolyzer capex ($0.056–0.1 kW h−1, $400–1000 kW−1) | Solar power carbon intensity (25–48 g CO2 kW h−1) |
| Nuclear S–I cycle | LCOH (literature range $1.47–2.71 kg−1 H2) | LCE literature range (0.41–2.2 kg CO2 kg−1 H2) |
| Nuclear Cu–Cl cycle | LCOH (literature range $1.47–2.70 kg−1 H2) | LCE literature range (0.7–1.8 kg CO2 kg−1 H2) |
| Electrolysis – nuclear | LCOE & electrolyzer capex ($0.08–0.12 kW h−1, $400–1000 kW−1) | Nuclear power carbon intensity (8.4–18 g CO2 kW h−1) |
 | ||
| Fig. 5 LCCM results for technology specific variations to LCOH and LCE values as detailed in Table 5. * LCOH parameter variations show are the combined variation of LCOE and electrolyzer capital costs as per the ranges in Table 5. Technologies highlighted by red text represent technologies with TRL levels of 5 or less. | ||
The LCCM results shown in Fig. 4 and 5 show a clear division of 0.4–1 orders of magnitude between technologies that utilise naturally-occurring energy-dense fuel and electrolysis routes that use less energy dense sources with more complex supply chain routes. The high LCCM values for electrolysis routes, despite very low LCE, are due to the low overall efficiencies created by multiple conversion steps resulting in very high LCOH values. The lowest LCCM routes are nuclear based thermochemical cycles, followed by biomass gasification and methane pyrolysis. The sensitivity of the LCCM values to changes in SMR reference values do not change the relative orders of magnitude of mitigation costs. For biomass gasification with CCS, despite the significantly higher cost the large reduction in LCE values results in small variations to the LCCM for the changes in the reference SMR values. It is interesting to highlight technologies of lower TRL have the lowest LCCM values.
The LCCM is an estimate of the additional cost associated with reducing the emissions of hydrogen production per tonne of CO2e against emissions of hydrogen production using SMR. It is important to recognize that LCCM reveals the lowest costs associated with any emission reduction, where lower proportional emission reductions are typically much less costly. The proportional reduction in emissions and cost increase relative to SMR is shown in Fig. 6, along with an indication of the variability in emissions and costs from the ranges defined in this study. Fig. 6 uses the ranges from Tables 3 and 4 for each technology. An emissions reduction of 100% represents a reduction in emissions equivalent to the life cycle emissions of unabated SMR, or in other words a net zero life cycle emission. The only technology considered capable of exceeding this limit to produce negative emissions is biomass gasification with CCS. Technologies on the lower end of the cost increase scale represent more cost-effective mitigation options, but deeper decarbonisation options require much larger costs.
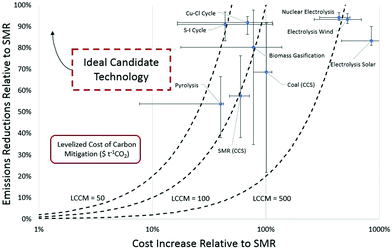 | ||
| Fig. 6 Proportional reduction in emissions against percentage cost increase relative to SMR. The variability of emissions and cost parameters shown reflect the full ranges of emissions and costs values used in this study and presented in Table 5. Biomass with CCS, emissions reduction of 213% and a cost increase of 168%, has been omitted from the chart as an outlier to allow focus on other technologies. | ||
4.3. Fossil fuel routes
The sensitivity range of decarbonization fractions presented in Fig. 6 shows under current practices all of the fossil-based routes do not exceed 65% decarbonization (76%, 69% and 92% best case for SMR CCS, pyrolysis and surface minded coal with CCS respectively). These decarbonization fractions are not commensurate with the decarbonization targets required in transport, heat and industry under many scenarios, particularly as global aspirations turn to net zero emissions in the second half of the 21st century.84 This highlights that technologies such as carbon capture and sequestration may potentially be an expensive exercise in heroic futility if all aspects of hydrogen supply chains are not addressed. No cost is currently applied to methane emissions and the costs of mitigating supply chain emissions are less well understood than emissions at the point of conversion. It has been suggested that 75% of global methane emissions from oil and gas supply chains are mitigatable, with half of these achieved at a positive net present value.85 Yet, the fact that these emissions remain suggests either there are hidden additional costs or that industry are not acting cost-optimally. With enough incentive (e.g. via pollution tax as per ref. 86), there is potential to constrain emissions considerably, as this study demonstrates under a low supply chain emissions scenario (and 90% capture rates) a LCE of 2.97 kg CO2 kg−1 H2: an 76% reduction from the median estimate of SMR without CCS.The supply chain emissions ranges presented in this study result in a higher upper estimate LCE values for SMR, SMR with CCS, methane pyrolysis and coal gasification with CCS than reported elsewhere in the literature. The deployment of CCS at-scale is the only method widely considered to be available to substantially mitigate CO2 emissions from fossil fuels, which so far has failed to meet ambitious expectations, and continued delays of demonstration projects are causing considerable uncertainty about the role CCS will play in carbon mitigation.87 This study has shown that equipping CCS to SMR (90% capture) only effectively reduces the life cycle GHG footprint of hydrogen production by 38–76%, depending on the contribution of supply chain emissions. Additionally, the capture rates may currently be lower than 90%.39 And whilst 90% or 95% capture is achievable, it is not guaranteed thus emissions may be even higher. The additional contributions to the overall GHG footprint from the supply chain are clearly significant, and decarbonization comparisons need to focus on more than just point-source emissions.
The cost of equipping CCS to SMR operations is highly variable (Fig. 1), with actual process costs by the few demonstration plants not publicly available. The operational and capital costs would also change with different capture rates, with higher capture rates requiring greater fuel duty and equipment.88 In Fig. 5, the sensitivity bounds of the CAC are varied by ±20% of the $96 t−1 CO2 (point-source cost) used in this study, resulting in a range of CAC from $78–118 t−1 CO2 (Fig. 4 and 5). It is likely that in the absence of an effective price on GHG emissions (in the form of carbon taxation or emissions trading schemes) there will be no economic incentive for decarbonizing hydrogen supply from SMR operations and as such no reductions in avoidance costs. As the business case for CO2 abatement is quite different to that of CO2 enhanced oil recovery or enhanced coal bed methane, these applications have not been considered here. A detailed review of carbon capture and utilization options is presented by Hunt et al.89 The CO2 storage potential to such economic uses, in any case, is small relatively to depleted oil and gas fields and saline aquifers.73
Amine-based CO2 absorption systems are also commercially mature technologies in the chemicals sector.90 Without a step-change in separation technologies, the cost of MEA-based CO2 separation is unlikely to contribute significantly to future cost reductions for CCS. Several proposals for equipping CCS to industrially active clusters with shared transport and storage infrastructure have been proposed and it is widely agreed the largest cost reductions can be achieved here.91–95 The transport and storage costs used in this study ($22 t−1 CO2) correspond to approximately 22% of the overall CCS cost for SMR and as such represent a potentially achievable cost reduction. However, it should be noted that the storage capacity of CO2 is a factor, of which the cheapest storage reservoirs also contribute the least to total available capacity.73 Long term monitoring of storage sites is also required to ensure leakage rates to the atmosphere of less 0.1% year−1 needed to ensure effective climate change abatement are maintained.96,97 Commercial scale storage sites will also have to manage the risks of financial penalties for unforeseen leakages.98 These ongoing costs are excluded from this study but may be non-negligible over time.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
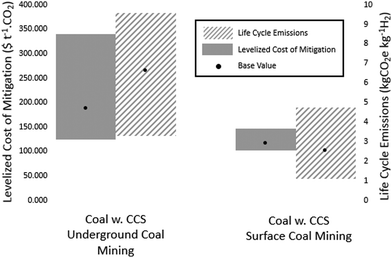
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H ratio in coal would suggest the higher quantity of CO2 per unit hydrogen should be costlier to sequester, which is not apparent from the lower CAC of ∼$65 t−1 CO2. There are two main factors contributing to the LCCM values obtained in this study. First, coal gasification for hydrogen production is commercially competitive with SMR where the gas prices are high or economies of scale are present. The studies concerning CAC for CCS of coal-to-hydrogen plants are large-scale facilities, distributing CCS capital across larger production volumes. Coupled with very low feedstock costs, the reduced capital burden per tonne of hydrogen product skews the CAC for equipping CCS. Secondly, CAC are associated with costs of reducing CO2 emissions from within a process facility and do include emissions from supply chain networks included here. Fig. 7 shows the influence of the coal supply chain on the overall LCCM and total LCE of hydrogen production from coal gasification with CCS (90% capture).
H ratio in coal would suggest the higher quantity of CO2 per unit hydrogen should be costlier to sequester, which is not apparent from the lower CAC of ∼$65 t−1 CO2. There are two main factors contributing to the LCCM values obtained in this study. First, coal gasification for hydrogen production is commercially competitive with SMR where the gas prices are high or economies of scale are present. The studies concerning CAC for CCS of coal-to-hydrogen plants are large-scale facilities, distributing CCS capital across larger production volumes. Coupled with very low feedstock costs, the reduced capital burden per tonne of hydrogen product skews the CAC for equipping CCS. Secondly, CAC are associated with costs of reducing CO2 emissions from within a process facility and do include emissions from supply chain networks included here. Fig. 7 shows the influence of the coal supply chain on the overall LCCM and total LCE of hydrogen production from coal gasification with CCS (90% capture).
The supply chain emissions from coal mining are only small for shallow mined coals, only representing a significant portion of total LCE when high CO2 capture rates (>85%) are achieved. The differences reported for surface mined coal are within the uncertainty ranges of the effectiveness and cost of coal gasification with CCS. For coal gasification with CCS (90% capture), decarbonization percentages of 58–92% 22–75% relative to SMR without CCS are achievable, for overground and underground mined coal respectively. For underground mined coal it is apparent that previous hydrogen production assessments have underestimated the potential contribution of the supply chain to overall LCE. It should be noted however, there is a large body of literature that suggests up to 60% of coal mine methane mitigation is achievable at relatively low cost.99,100 The low sensitivity of LCOH to coal prices suggest there is potential to reduce the 4.05 kg CO2e kg−1 H2 for underground mines closer to the surface mining value of 0.45 kg CO2e kg−1 H2 without a significant increase in the LCOH. Other than minimizing surface area, there are currently no available options for reducing fugitive methane emissions from open-surface mines. For CCS with 90% capture and 55% HHV overall efficiency, the central estimate of 2.1 kg CO2e kg−1 H2, from surface mined coal is the lowest LCE of any of the fossil fuel routes presented.
Coal-to-hydrogen processes are however less prevalent as dedicated hydrogen producers, with advanced coal gasification concepts aiming to integrate CO2 separation with water–gas shift reactions to achieve higher process efficiencies with ease of CO2 capture.101 Whilst reduction of CCS costs may be achievable with technological development, the order of magnitude is entirely speculative. Like SMR with CCS, the integration of industrial clusters with shared transport and storage infrastructure may provide future cost reductions. The order of magnitude estimates for coal-to-hydrogen with CCS are likely to remain around the $60–100 t−1 CO2.47
 | ||
| Fig. 8 Sensitivity of the methane pyrolysis LCCM to major LCOH and LCE variables used in this study. | ||
The most sensitive factor for pyrolysis economics (and thus the LCCM) is the product value assigned to carbon produced (Fig. 8). The large-scale implementation of pyrolysis processes for global decarbonization efforts would generate massive quantities of carbon of which the only solid-manufactured product used in comparable volumes today is concrete. Assigning value to the carbon products (>$∼170 t−1 carbon) makes pyrolysis more economically competitive than SMR at $4 GJ−1 gas prices and the LCCM zero. At gas prices of approximately $2 GJ−1 pyrolysis becomes more economically competitive than SMR with carbon values exceeding $90 t−1 carbon. The physical and electrical properties of the solid carbon will strongly determine its value as a co-product. Initial process developments will likely find receptive markets for high value carbon products including graphite for lithium-ion batteries, carbon fibers for reinforced composite materials or needle coke for graphite electrodes in electric arc furnaces. Catalysed decomposition has in the past been investigated primarily for this reason.67 An excellent summary current available carbon markets and relative sizes is presented in a recent review by the Argonne National Laboratory.66
Using current practices, methane pyrolysis and natural gas as a heat source only achieves a decarbonization fraction of 38–67%. Alternatively, direct emissions from pyrolysis could be reduced to zero by using nuclear as a heat source, or by burning ∼35% of the hydrogen product to supply the necessary heat.70 This however would further increase the required capital and natural gas utilization, adding an additional $40 t−1 CO2 to the LCCM despite the reduced LCE emissions. The LCE range of 4.2–9.14 kg CO2 kg−1 H2 for methane pyrolysis presented in this study identified a central estimate of 6.1 kg CO2 kg−1 H2, which is higher than any LCE value for pyrolysis reported elsewhere. This decarbonization fraction (∼54% reduction compared to SMR without CCS) may be insufficient in western economies with ambitious carbon mitigation targets. This finding is of particular importance as the technology is regularly cited in academic literature as a means of producing CO2-free hydrogen,2,4,6,9,14,19,21,66,68–70,102–106 which is only achievable if supply chain emissions are low. It should be noted, however, in contrast to technologies such as CCS the storage of a solid by-product carbon is relatively simple either temporarily or permanently, an idea first proposed as the ‘carbon moratorium’ by Kreysa.104 Storage of solid carbon, or the ‘reverse carbon chain’, may provide a practical, low-cost carbon sequestration solution for locations where gaseous CO2 storage sites are limited (or non-existent) or expensive. There may also be an advantage in storing the carbon in an easily recoverable form pending future technological developments.
Given that pyrolysis processes have had little commercial development for hydrogen applications, it is anticipated that capital costs will be significantly reduced through research, innovation and deployment at scale. There are several technical challenges associated with management of the solid carbon produced which fouls (cokes) solid catalysts.102,107–109 However, proponents of the technology claim these limitations can be overcome with appropriate process and reactor design to allow easy separation of the carbon product.10,52,106,110 If pursued as a cost-effective mitigation option, innovation in reactor design, carbon removal schemes and high temperature processing equipment could substantially enhance cost competitiveness. Reactor designs that can facilitate carbon separation and achieve high conversion will be critical for commercial pyrolysis process.
4.4. Electrolysis routes
Despite the low LCE of electrolysis routes, the overall LCCM for each route is 0.4–1 orders of magnitude higher. For wind and solar electrolysis, the intermittent nature of power supply results in low-utilization of high electrolyzer capital, increasing the LCOH values. The full LCCM range is $271–707 t−1 CO2 (base $534 t−1 CO2) and $529–1233 t−1 CO2 (base $973 t−1 CO2) for wind and solar electrolysis respectively. For nuclear electrolysis, the high utilization factor minimizes the LCOH sensitivity to electrolyser capital and the LCOE dominates the LCOH. This results in a full LCCM range of $298–556 t−1 CO2 (base $443 t−1 CO2) for nuclear electrolysis. The sensitivity bounds in Fig. 5 show the full combined range of electrolyzer capital and LCOE variations for each route. The individual LCOE and electrolyzer capital contributions to the LCOH are shown via a sensitivity analysis in Fig. 9.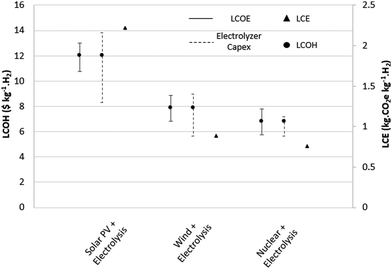 | ||
| Fig. 9 Sensitivity of electrolysis routes to supplied LCOE, electrolyzer capital cost and emissions intensity of the power supply. Utilization rates and cost inputs are summarized in Table 2 and economic assumptions in Tables S9–S12 in the ESI.† | ||
The LCOH values estimated in this study are higher than the ranges presented in the literature. Given the capital-intensive nature of renewable power generation and the fact that short-run marginal operating costs are low-to-zero, the overall system efficiency and weighted average cost of capital (discount rate) used in project evaluations have a critical impact on project economics.110 The standard capacity factors used for each technology will likely show significant regional variations, drastically altering the realized LCOH. The capacity factors presented here represent generic installations. It should be noted however, the weighted average cost of capital (10%) used in this study is consistent with other techno-economic hydrogen production studies achieving similar hydrogen production costs.111
It is interesting to note that electrolysis avoidance costs in their current status are comparable to negative emissions technologies such as direct capture, estimated to be $600–700 t−1 CO2 avoided.111,112 This however does not reflect cost reductions available to electrolysis (in particular solar and wind capital cost reductions) pathways, which would require radically new designs for negative emissions technologies to reduce further. Electricity prices from solar and wind power are highly variable and largely depend on location specific factors. Electricity produced from solar is approximately proportional to solar irradiance and wind proportional to the cube of the wind speed. Effective deployment of each technology in high resource availability areas undoubtedly increases the resulting capacity factor. For example, wind capacity factors have increased markedly in the past decade and in some sites (e.g. Denmark) may exceed 50%.113 To reflect region-specific variability, Fig. 10(a) shows the influence of technology capacity factor using the current and future LCOE and electrolyzer costs used in this study. Note that this assumes the electrolyser is sized for maximum generation capacity, thus this capacity factor is the same as the generation capacity. The design of the electrolyser may be optimised to improve overall system efficiency if it were undersized, depending on the electricity generation profile. This is not considered here but may serve to moderately reduce total costs.
Under ideal conditions with optimistic future costs, the LCCM reaches minimum values of $200 t−1 CO2 and $300 t−1 CO2 for wind and solar respectively (Fig. 10(a)). It is possible to supply additional grid-based electricity to increase the electrolyzer capacity factor, but the carbon intensity of the grid supply strongly influences the LCE of the hydrogen produced. This is illustrated in Fig. 10(b), which shows the effect of increasing the utilization of an optimistic 50% capacity factor future wind electrolysis scenario using the average EU (∼0.275 kg CO2 kW h−1, $0.081 kW h−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 114,115) and French (∼0.050 kg CO2 kW h−1, $0.0596 kW h−1
114,115) and French (∼0.050 kg CO2 kW h−1, $0.0596 kW h−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 114,115) electricity grids. It is apparent from the increase in LCCM for the EU grid average that the decrease in LCOH for higher utilization factors is outweighed by the increased LCE of electricity production. Comparatively, use of the French grid supply consisting of approximately 75% nuclear power maintains the LCCM at approximately $200 t−1 CO2 as electrolyzer capacity factors approach 95%.
114,115) electricity grids. It is apparent from the increase in LCCM for the EU grid average that the decrease in LCOH for higher utilization factors is outweighed by the increased LCE of electricity production. Comparatively, use of the French grid supply consisting of approximately 75% nuclear power maintains the LCCM at approximately $200 t−1 CO2 as electrolyzer capacity factors approach 95%.
Despite this, wind and solar electrolysis routes are promising technologies for achieving highly decarbonized hydrogen supply (91–96% and 81–90%, respectively). Wind and solar power have witnessed substantial cost reductions over the past two decades and potential areas of future cost reduction have been identified by others.116 The ability of solar PV to meet the required cost reductions to be a more cost competitive mitigation option requires a balance between the investments required to produce and install a module, the total energy provided by that module and its lifetime and conversion efficiency.117 New materials realising sufficient efficiency, stability and cost will be required.118 Assuming such radical new designs and materials are possible, the performance and cost of the active components remain to be demonstrated simultaneously.17 Similarly, one of key drivers of the increasing competitiveness of wind power has been continued innovation in turbine design and operation. Continuous increases in the average capacity of turbines, hub-heights and swept areas have allowed higher utilization factors and reduced costs.119 Continued innovation in turbine-design leading to higher utilization factors is required to further reduce costs.
Cost reductions of a similar magnitude are also required in electrolyzer manufacturing as the future PEM electrolyzer costs of $400 kW−1 used in this study are substantially lower than today's costs.72 Cost decreases of this magnitude will require mass production of PEM electrolyzers with streamlined market integration, automated and standardized manufacturing processes with robust quality control methods for avoiding component failure during operation.72 However, based on the analysis presented, it is unlikely that future developments utilizing existing technological pathways will reach cost competitiveness as mitigation options using current technological pathways. To be cost competitive with fossil fuel mitigation costs and natural gas prices in the $3–6 GJ−1 range, this would require a LCOH of less than $3 kg−1 H2 for wind electrolysis and less than $2.50 kg−1 H2 for solar electrolysis with current embodied emissions ranges. Reducing the LCOH using grid electricity only achieves relatively constant mitigation costs (∼$200 t−1 CO2) with increasing utilization factors for the best-case wind electrolysis scenario (50% capacity factor) if the grid-supply is less carbon intense than the generation technology or if low-cost nuclear power is available. This highlights that for a highly decarbonized hydrogen supply via electrolytic routes commensurate with demand profiles of current hydrogen applications, low-cost nuclear power is required.
Whilst LCOH and LCE associated with the nuclear electrolysis option are lower than those from wind and solar PV, the additional conversion steps and capital for nuclear electrolysis routes compared to thermochemical cycles substantially increases the LCOH, resulting in an average LCCM of $455 t−1 CO2. Where multiple conversion steps are involved, the overall efficiency of the process is the product of the individual conversion efficiencies. Thermal efficiencies of nuclear and combustion power plants to electricity operating through Rankine cycles range from 31% for a Magnox type to around 40% for an advanced gas cooled reactor.120 Using a 35% reactor conversion efficiency and combining this with the optimistic 85% electrolyser efficiency used in this study, the overall efficiency of nuclear electrolytic routes is 29.75%. Using optimistic future costs of $400 kW−1 electrolyzer costs and the lower bound LCOE of $0.08 kW h−1, the LCCM of nuclear electrolysis is reduced to ∼$300 t−1 CO2.
Government policy may further support regulatory structures to enhance project viability by minimising the cost of debt of renewable-based projects. Alternatively, regulatory structures to support business models leading to a high penetration of renewables with lengthy periods of excess electricity generated that would otherwise be wasted could enhance utilization factors of electrolyzers at no cost. In either case, both mechanisms require policy intervention to support one technological pathway over others. Such interventions may be required to achieve very high decarbonization fractions in the future, but initial investments should focus where the highest return on investment (dollars spent per tonne of carbon avoided) are achieved. To reduce costs in line with current production methods they require higher capacity factors, lower electrolyzer costs and/or low-cost low-carbon electricity supply likely provided by nuclear. Nevertheless, hydrogen production from decentralized electrolysis will likely find cost-competitive decarbonization applications in the future, reflecting a scenario-based constraint that some hydrogen demand is in areas that cannot be sensibly accessed by centrally produced hydrogen networks.
4.5. Nuclear thermochemical cycles
From Fig. 5, the direct conversion of nuclear heat from power plants to hydrogen via the two thermochemical cycles discussed are promising candidate technologies for low mitigation costs and high decarbonization fractions. The LCCM of the S–I and Cu–Cl cycles of $46 t−1 CO2 and $72 t−1 CO2 respectively are the lowest base-case values obtained. Although the thermochemical cycle efficiencies (∼50%) have yet to be demonstrated at scale, it has been the subject of significant research by the Japan Atomic Energy Agency,121 General Atomics122 and Westinghouse.123 The full range of LCOH and LCE values reported in the literature generate LCCM ranges of $17–120 t−1 CO2 and $117–118 t−1 CO2 for the S–I and Cu–Cl cycles respectively.Nuclear thermochemical cycles and nuclear electrolysis all achieve decarbonization fractions in excess of 90% compared to SMR without CCS. It is important to note, however, that no capital costs were reported in the studies comparing the LCOH and LCE of the two thermochemical cycles investigated, which have been shown to be highly location specific. Although unit costs for technologies usually decrease with increasing volume of production, nuclear power has consistently seen the opposite within the United States; which reflects the idiosyncrasies of the regulatory environment as public opposition grew and regulations were tightened.124 Lovering et al.125 reviewed global historical construction costs of nuclear reactors and found in contrast to the rapid U.S. cost escalations, much milder cost escalations and in some cases cost declines existed elsewhere. They concluded that there is no inherent cost escalation trend associated with nuclear technology, and the large variance witnessed in cost trends over time and across different countries, even with similar nuclear reactor technologies, suggests that cost drivers other than learning-by-doing have dominated the cost experience of nuclear power construction.125 Additionally, as the majority of literature on nuclear power costs have focussed almost exclusively on the United States and France, there is an incomplete picture of the economic evolution of the technology.125
The true production costs of hydrogen via nuclear thermochemical cycles and nuclear electrolysis will likely be largely uncertain until political rhetoric surrounding nuclear power subsides and scientists and engineers are free to innovate in this sector. Nonetheless, nuclear thermochemical cycles as energy dense, highly decarbonized energy sources are promising routes provided an actual LCOH of around $2 kg−1 with current embodied emissions values is achievable. In particular, the Cu–Cl may be capable of using low-grade waste heat from nuclear reactors after power generation, increasing the value proposition of nuclear reactors.
4.6. Biomass gasification
Biomass gasification appears to be an effective and relatively cheap means of carbon mitigation from hydrogen production. The calculated LCCM from literature values range from $68–85 t−1 CO2 and $21–213 t−1 CO2 biomass gasification with and without CCS respectively. The large variability of unabated biomass gasification stems from the large spread of embodied emissions from biomass cultivation and capital cost of the facilities. The very low or negative LCE footprint results in a high degree of decarbonization (Fig. 5) relative to SMR. The low range for biomass gasification with CCS reflects a ±20% of the values obtained for the single study available.Despite the high decarbonization fraction of biomass gasification routes, the current concept of biomass-to-hydrogen has several limitations. Only a very small percentage of solar energy is converted to hydrogen (∼6–12 wt% H2 kg−1 biomass126), meaning that large land requirements for energy crops are necessary to contribute significantly to the existing hydrogen demand (or potential future hydrogen uses). Biomass energy crops may also be geographically constrained to areas not in competition with food production or to the utilization of agricultural waste. For example, a hydrogen yield of 7.97 wt% from purpose grown energy crops (4.49 dry tonnes acre−1) would require ∼48% of the U.S. agricultural crop area of 545![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 square miles to satisfy current global hydrogen demand of 60 million tonnes per annum.22 This number would likely grow substantially if other sectors such as heat and transport were decarbonized with H2 supply. The costly transportation of large quantities of dispersed, low-energy density biomass to central processing plants is another one of the major issues of the technology. Furthermore, the environmental impact from the significant demand on land, nutrient supply, fertilizers and water for bioenergy crops would reduce the environmental benefits of such an approach.
000 square miles to satisfy current global hydrogen demand of 60 million tonnes per annum.22 This number would likely grow substantially if other sectors such as heat and transport were decarbonized with H2 supply. The costly transportation of large quantities of dispersed, low-energy density biomass to central processing plants is another one of the major issues of the technology. Furthermore, the environmental impact from the significant demand on land, nutrient supply, fertilizers and water for bioenergy crops would reduce the environmental benefits of such an approach.
The utilization of waste biomass or waste agricultural resources may increase the economic and environmental benefits in the displacement of landfill. A recent report127 suggests with anticipated improvements in agricultural practices and plant breeding, feedstocks may exceed 244 million dry tons at a farm-gate price of $60 dry ton−1. It has also been proposed that waste biomass feedstocks could be co-fired in coal gasification facilities to further reduce the net CO2 closer to zero with minimal impact on the downstream flue gas treatment unit.128 In a plant with post-combustion capture this increases the cost of electricity by 6% and has no impact on the cost of CO2 avoidance, but the cost depends strongly on the cost of biomass.128 While research and development focuses on gasification, synergies with other fuel production process (biofuels) may accelerate the rate of market uptake should biomass be pursued.3
The production of hydrogen from biomass with CCS is one of only a few technologies that may deliver negative emissions at relatively modest costs, which may become important for global decarbonization issues in the second half of this century.129 But basic feedstocks are location-limited and competition with other more efficient liquid biofuel routes and direct power generation from waste feedstocks may restrict the role biomass-to-hydrogen routes fulfil. This is reflected by several commercial examples of biomass for heat and power130 but no completed industrial-scale demonstrations of any biomass technology for hydrogen production.126 The relative paucity of studies quantifying costs and emissions drives the relatively small ranges for biomass gasification with CCS when compared to the larger ranges of estimates for other technologies seen in Fig. 6. A challenge for future research is to better quantify all parameters relating to biomass gasification with CCS costs and emissions.
5. Conclusions
As with all comparisons between fossil routes and renewables, cost and emissions data are frequently misused by advocates of all parties to push policy-makers and public opinion further along the polarizing debate of the role of fossil fuels in a low-carbon system. The best approach to decarbonizing hydrogen supply at least cost is not to champion or demonize specific technologies, but to jointly provide evidence to policy-makers to support higher levels of climate ambition. Ultimately, the development of low-CO2, large-scale and economically competitive hydrogen production processes is fundamental to the production of low-carbon fuels, fertilizers and other petrochemicals. To achieve this, there is a significant amount of research going on to improve the performance of existing methods and to find new promising routes to generate hydrogen.In this work, technical, economic and environmental aspects of 12 different hydrogen production techniques were evaluated and compared using the LCCM and decarbonization fraction. Production costs and life cycle emissions were parameterised and re-estimated from currently available estimates, producing robust ranges to describe the uncertainties for each technology. The LCCM and decarbonization fraction was utilised as a basis for providing an overall measure of the additional costs required to decarbonize hydrogen supply for any future uses. Such an overview provides a clearer picture of the relative merits of various pathways than an isolated measure and the outcomes of this research are summarized presently.
There is broadly a trade-off between the cost of mitigation and the proportion of decarbonization achieved. The cheapest methods of decarbonization are via the fossil routes due to their low cost of extraction and processing, but they only offer moderate decarbonisation levels, with a largest central estimate reduction of 69% from surface mined coal with CCS (90% capture). There is an order of magnitude difference in LCCM between renewable electrolytic routes and fossil-based hydrogen production, suggesting that to decarbonize hydrogen supply for current and potential future uses, cost-effective low-CO2 bridging technologies that take advantage of fossil-feedstocks may be required. This will allow the necessary time for the required infrastructure and end-use applications to sustain a large penetration of hydrogen into the energy economy to be developed and minimize cost barriers for renewable-based technologies.
However, emissions associated with the fossil fuel routes may be higher than previously considered due to an underestimation of upstream emissions to the overall footprint. Emissions from supply chains vary significantly and are non-negligible, particularly methane emissions across natural gas and coal supply chains. The sensitivity range of decarbonization fractions (Fig. 6) showed central estimates under current practices for all of the fossil-based routes do not exceed 69% decarbonization (76%, 67% and 92% best cases for SMR CCS, pyrolysis and surface minded coal with CCS respectively). These decarbonization fractions are not commensurate with the decarbonization targets required in transport, heat and industry under many scenarios, particularly as global aspirations turn to net zero emissions in the second half of the 21st century in line with the Paris Agreement.84 This highlights that technologies such as carbon capture and sequestration may potentially be an expensive exercise in heroic futility if all aspects of hydrogen supply chains are not addressed. No cost is currently applied to methane emissions and the costs of mitigating supply chain emissions are less well understood than emissions at the point of conversion. This remains an important area for future research.
Methane pyrolysis, however, may be the most cost effective short-term mitigation solution that encourages building infrastructure necessary to sustain a high penetration of hydrogen into the energy economy. Its decarbonization fraction is heavily dependent on managing the contribution of supply chain emissions, whilst cost-effectiveness will be governed by the price of solid-carbon product and innovation in reactor designs. The LCE range (4.2–9.14 kg CO2 kg−1 H2) presented in this study identified a median value of 6.1 kg CO2 kg−1 H2, which is higher than any LCE value for pyrolysis reported elsewhere in academic literature. This finding is of particular importance as the technology is regularly cited in academic literature as a means of producing CO2-free hydrogen,2,4,6,9,14,19,21,66,68–70,102–106 which is only achievable if supply chain emissions are low.
Whilst electrolysis routes offer significantly higher emissions reductions than fossil routes (80–95% compared to SMR without CCS), costs are 0.4–1 orders of magnitude higher. It is interesting to note that hydrogen from electrolysis avoidance costs in their current status are comparable to negative emissions technologies such as direct capture, estimated to be $600–700 t−1 CO2 avoided. Production routes are more complex than those that utilise a naturally-occurring energy-dense fuel and electrolyser costs are high at modest capacity factors. They may indeed play an important role in deep decarbonization pathways (especially in the absence of negative emissions technologies), but to reduce costs in line with current production methods they require higher capacity factors, lower electrolyser costs and/or a low-cost nuclear electricity supply. For cost-effective hydrogen commensurate with current demand profiles, low-cost, low-carbon and reliable electricity supply is essential. Government policy may further support regulatory structures to enhance project viability by minimising the cost of debt of renewable-based projects. Alternatively, regulatory structures to support business models leading to a high penetration of renewables with lengthy periods of excess electricity generated that would otherwise be wasted could enhance utilization factors of electrolyzers at no cost. In either case, both mechanisms require policy intervention to support one technological pathway over others. Such interventions may be required to achieve very high decarbonization fractions, but initial investments should focus where the highest return on investment (dollars spent per tonne of carbon avoided) are achieved.
Nuclear thermochemical cycles appear to be highly cost-effective mitigation options but the political landscape surrounding nuclear utilisation is regionally varied and severely restricted due to a lack of public approval and political creativity. Additionally, the supporting data lacks depth and transparency and should be considered speculative at best. Further work is needed to demonstrate thermochemical cycle efficiencies and cost at-scale. For hydrogen without fossil fuels, low-cost nuclear is required to minimize cost. This therefore remains an important research priority.
Biomass gasification offers a very high decarbonization potential (∼80% without CCS, compared to SMR without CCS) and is one of the few routes to potentially achieve negative emissions at relatively low cost. This may become important in the second half of this century as the drive to deeply decarbonize further increases. But despite this, it may continue to represent a small contribution to hydrogen production due to limited resource availability, relatively low conversion efficiencies and competition for agricultural land required for any substantial market penetration. The relative paucity of studies quantifying costs and emissions remains a challenge for future research to better quantify all parameters relating to biomass gasification, in particular the costs and emissions when coupled with CCS.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Brett Parkinson would like to thank the generous support of the General Sir John Monash Foundation. Funding for the Sustainable Gas Institute is gratefully received from Royal Dutch Shell, Enágas SA, and from the Newton/NERC/FAPESP Sustainable Gas Futures project NE/N018656/1. Note that funding bodies were not involved in the implementation or reporting of this study.References
- M. Hulme, Nat. Clim. Change, 2016, 6, 222 CrossRef.
- N. Z. Muradov and T. N. Veziroğlu, Int. J. Hydrogen Energy, 2008, 33, 6804–6839 CrossRef CAS.
- G. Simbollotti, IEA Energy Technology Essentials-Hydrogen Production and Distribution, 2017 Search PubMed.
- A. Konieczny, K. Mondal, T. Wiltowski and P. Dydo, Int. J. Hydrogen Energy, 2008, 33, 264–272 CrossRef CAS.
- A. H. Fakeeha, A. A. Ibrahim, W. U. Khan, K. Seshan, R. L. Al Otaibi and A. S. Al-Fatesh, Arabian J. Chem., 2018, 11, 405–414 CrossRef CAS.
- U. P. M. Ashik, W. M. A. Wan Daud and H. F. Abbas, Renewable Sustainable Energy Rev., 2015, 44, 221–256 CrossRef CAS.
- M. Granovskii, I. Dincer and M. A. Rosen, J. Power Sources, 2006, 157, 411–421 CrossRef CAS.
- N. Muradov, Int. J. Hydrogen Energy, 2017, 42, 14058–14088 CrossRef CAS.
- N. Muradov and T. Veziroǧlu, Int. J. Hydrogen Energy, 2005, 30, 225–237 CrossRef CAS.
- L. Weger, A. Abánades and T. Butler, Int. J. Hydrogen Energy, 2017, 42, 720–731 CrossRef CAS.
- B. C. R. Ewan and R. W. K. Allen, Int. J. Hydrogen Energy, 2005, 30, 809–819 CrossRef CAS.
- C. Acar and I. Dincer, Int. J. Hydrogen Energy, 2014, 39, 1–12 CrossRef CAS.
- I. Dincer and C. Acar, Int. J. Hydrogen Energy, 2015, 40, 11094–11111 CrossRef CAS.
- O. Machhammer, A. Bode and W. Hormuth, Chem. Eng. Technol., 2016, 39, 1185–1193 CrossRef CAS.
- J. Speirs, P. Balcombe, E. Johnson, J. Martin, N. Brandon and A. Hawkes, Energy Policy, 2018, 118, 291–297 CrossRef.
- A. Haryanto, S. Fernando, N. Murali and S. Adhikari, Energy Fuels, 2005, 19, 2098–2106 CrossRef CAS.
- J. W. Ager, M. R. Shaner, K. A. Walczak, I. D. Sharp and S. Ardo, Energy Environ. Sci., 2015, 8, 2811–2824 RSC.
- J. R. McKone, N. S. Lewis and H. B. Gray, Chem. Mater., 2014, 26, 407–414 CrossRef CAS.
- N. Z. Muradov and T. N. Veziroğlu, Carbon-neutral fuels and energy carriers, CRC Press, 2011 Search PubMed.
- The Royal Society, Options for producing low-carbon hydrogen at scale, Report DES4801_2, The Royal Society, 2018.
- B. Parkinson, M. Tabatabaei, D. C. Upham, B. Ballinger, C. Greig, S. Smart and E. McFarland, Int. J. Hydrogen Energy, 2018, 43, 2540–2555 CrossRef CAS.
- National Research Council, The hydrogen economy: opportunities, costs, barriers, and R&D needs, Report 0309091632, National Academies Press, 2004.
- D. Gray and G. Tomlinson, Mitretek Technical Paper MTR, 2002, 31, 2002.
- F. Mueller-Langer, E. Tzimas, M. Kaltschmitt and S. Peteves, Int. J. Hydrogen Energy, 2007, 32, 3797–3810 CrossRef CAS.
- S. Penner, Energy, 2006, 31, 33–43 CrossRef CAS.
- A. H. Strømman and E. Hertwich, Hybrid life cycle assessment of large-scale hydrogen production facilities, 2004.
- J. Ruether, M. Ramezan and E. Grol, Life-cycle analysis of greenhouse gas emissions for hydrogen fuel production in the United States from LNG and coal, DOE/NETL-2006/1227, November, 2005.
- R. Bhandari, C. A. Trudewind and P. Zapp, J. Cleaner Prod., 2014, 85, 151–163 CrossRef CAS.
- J. Lane and P. Spath, Technoeconomic analysis of the thermocatalytic decomposition of natural gas, National Renewable Energy Lab., Golden, CO (US), 2001 Search PubMed.
- E. Cetinkaya, I. Dincer and G. F. Naterer, Int. J. Hydrogen Energy, 2012, 37, 2071–2080 CrossRef CAS.
- C. Koroneos, A. Dompros, G. Roumbas and N. Moussiopoulos, Int. J. Hydrogen Energy, 2004, 29, 1443–1450 CrossRef CAS.
- D. Sadler, H21 Leeds City Gate Report, Northern Gas Networks, 2016 Search PubMed.
- J. Dufour, D. P. Serrano, J. L. Gálvez, A. González, E. Soria and J. L. Fierro, Int. J. Hydrogen Energy, 2012, 37, 1173–1183 CrossRef CAS.
- L. Tock and F. Maréchal, Int. J. Hydrogen Energy, 2012, 37, 11785–11795 CrossRef CAS.
- J. Dufour, D. P. Serrano, J. L. Gálvez, J. Moreno and C. García, Int. J. Hydrogen Energy, 2009, 34, 1370–1376 CrossRef CAS.
- M. Melania, Current central hydrogen production from natural gas without CO2 sequestration version 3.101.
- G. Collodi and F. Wheeler, Chem. Eng. Trans., 2010, 19, 37–42 Search PubMed.
- G. Collodi, G. Azzaro, N. Ferrari and S. Santos, Energy Procedia, 2017, 114, 2690–2712 CrossRef CAS.
- P. Balcombe, J. Speirs, E. Johnson, J. Martin, N. Brandon and A. Hawkes, Renewable Sustainable Energy Rev., 2018, 91, 1077–1088 CrossRef CAS.
- R. Kothari, D. Buddhi and R. L. Sawhney, Renewable Sustainable Energy Rev., 2008, 12, 553–563 CrossRef CAS.
- N. V. S. N. M. Konda, N. Shah and N. P. Brandon, Int. J. Hydrogen Energy, 2011, 36, 4619–4635 CrossRef.
- C. E. G. Padro and V. Putsche, Survey of the economics of hydrogen technologies, National Renewable Energy Lab., Golden, CO (US), 1999 Search PubMed.
- Royal Belgian Academy Council of Applied Science, Hydrogen as an energy carrier, 2006.
- P. Chiesa, S. Consonni, T. Kreutz and R. Williams, Int. J. Hydrogen Energy, 2005, 30, 747–767 CrossRef CAS.
- P. Burmistrz, T. Chmielniak, L. Czepirski and M. Gazda-Grzywacz, J. Cleaner Prod., 2016, 139, 858–865 CrossRef CAS.
- A. Verma and A. Kumar, Appl. Energy, 2015, 147, 556–568 CrossRef CAS.
- E. S. Rubin, J. E. Davison and H. J. Herzog, Int. J. Greenhouse Gas Control, 2015, 40, 378–400 CrossRef CAS.
- IEAGHG, Co-production of hydrogen and electricity by coal gasification with CO2 capture–updated economic analysis, 2008.
- P. Nikolaidis and A. Poullikkas, Renewable Sustainable Energy Rev., 2017, 67, 597–611 CrossRef CAS.
- GoGreenGas, BioSNG Demonstration Plant Project Close-Down Report, 2017.
- T. L. Group, Green Hydrogen from Biomass, https://www.linde-engineering.com/en/innovations/green_hydrogen_from_biomass/index.html, accessed April 2018.
- J. D. Holladay, J. Hu, D. L. King and Y. Wang, Catal. Today, 2009, 139, 244–260 CrossRef CAS.
- D. Wang, S. Czernik, D. Montane, M. Mann and E. Chornet, Ind. Eng. Chem. Res., 1997, 36, 1507–1518 CrossRef CAS.
- J. R. Bartels, M. B. Pate and N. K. Olson, Int. J. Hydrogen Energy, 2010, 35, 8371–8384 CrossRef CAS.
- J. Yao, M. Kraussler, F. Benedikt and H. Hofbauer, Energy Convers. Manage., 2017, 145, 278–292 CrossRef CAS.
- A. Mehmeti, A. Angelis-Dimakis, G. Arampatzis, S. J. McPhail and S. Ulgiati, Environments, 2018, 5, 24 CrossRef.
- A. Valente, D. Iribarren and J. Dufour, Int. J. Life Cycle Assess., 2017, 22, 346–363 CrossRef CAS.
- M. Martín-Gamboa, D. Iribarren, A. Susmozas and J. Dufour, Bioresour. Technol., 2016, 214, 376–385 CrossRef PubMed.
- A. Susmozas, D. Iribarren and J. Dufour, Int. J. Hydrogen Energy, 2013, 38, 9961–9972 CrossRef CAS.
- Y. Kalinci, A. Hepbasli and I. Dincer, Int. J. Hydrogen Energy, 2012, 37, 14026–14039 CrossRef CAS.
- J. Moreno and J. Dufour, Int. J. Hydrogen Energy, 2013, 38, 7616–7622 CrossRef CAS.
- D. Iribarren, A. Susmozas, F. Petrakopoulou and J. Dufour, J. Cleaner Prod., 2014, 69, 165–175 CrossRef CAS.
- A. Susmozas, D. Iribarren, P. Zapp, J. Linβen and J. Dufour, Int. J. Hydrogen Energy, 2016, 41, 19484–19491 CrossRef CAS.
- D. Mahajan, C. E. Taylor and G. A. Mansoori, J. Pet. Sci. Eng., 2007, 56, DOE/NETL-IR-2007-089.
- A. M. Amin, E. Croiset and W. Epling, Int. J. Hydrogen Energy, 2011, 36, 2904–2935 CrossRef CAS.
- R. A. Dagle, V. Dagle, M. D. Bearden, J. D. Holladay, T. R. Krause and S. Ahmed, An Overview of Natural Gas Conversion Technologies for Co-Production of Hydrogen and Value-Added Solid Carbon Products, Pacific Northwest National Lab. (PNNL), Richland, WA (United States), Argonne National Lab. (ANL), Argonne, IL (United States), 2017 Search PubMed.
- N. Shah, D. Panjala and G. P. Huffman, Energy Fuels, 2001, 15, 1528–1534 CrossRef CAS.
- M. Steinberg, Int. J. Hydrogen Energy, 1999, 24, 771–777 CrossRef CAS.
- N. Z. Muradov, Energy Fuels, 1998, 12, 41–48 CrossRef CAS.
- B. Parkinson, J. W. Matthews, T. B. McConnaughy, D. C. Upham and E. W. McFarland, Chem. Eng. Technol., 2017, 40, 1022–1030 CrossRef CAS.
- S. Postels, A. Abánades, N. von der Assen, R. K. Rathnam, S. Stückrad and A. Bardow, Int. J. Hydrogen Energy, 2016, 41, 23204–23212 CrossRef CAS.
- S. M. Saba, M. Müller, M. Robinius and D. Stolten, Int. J. Hydrogen Energy, 2017, 43, 1209–1223 CrossRef.
- Zero Emissions Platform, The Costs of CO2-Storage – Post-Demonstration CCS in the EU, 2011.
- K. E. Ayers, C. Capuano and E. B. Anderson, ECS Trans., 2012, 41, 15–22 CAS.
- G. R. Saur, B. James and W. Colella, Hydrogen and fuel cells program: production case studies.
- J. C. Koj, A. Schreiber, P. Zapp and P. Marcuello, Energy Procedia, 2015, 75, 2871–2877 CrossRef CAS.
- M. Fischer, M. Faltenbacher and O. Schuller, Life Cycle Impact Assessment, ECTOS, 2005 Search PubMed.
- K. R. Schultz, M. B. Gorensek, J. Stephen, M. A. L. Herring, R. Moore, J. E. O’Brien, P. S. Pickard, B. E. Russ and W. A. Summers, Carbon-Neutral Fuels Energy Carriers, 2011, 203 Search PubMed.
- L. Brown, J. Funk and S. Showalter, Initial screening of thermochemical water-splitting cycles for high efficiency generation of hydrogen fuels using nuclear power, General Atomics, San Diego, CA (US), University of Kentucky, Lexington, KY (US), Sandia National Labs., Albuquerque, NM (US), 1999.
- M. T. Balta, I. Dincer and A. Hepbasli, Int. J. Hydrogen Energy, 2009, 34, 2925–2939 CrossRef.
- L. C. Brown, G. E. Besenbruch, R. Lentsch, K. R. Schultz, J. Funk, P. Pickard, A. Marshall and S. Showalter, High efficiency generation of hydrogen fuels using nuclear power, GENERAL ATOMICS (US), 2003 Search PubMed.
- Z. L. Wang, G. F. Naterer, K. S. Gabriel, R. Gravelsins and V. N. Daggupati, Int. J. Hydrogen Energy, 2010, 35, 4820–4830 CrossRef CAS.
- A. Ozbilen, I. Dincer and M. A. Rosen, Int. J. Hydrogen Energy, 2011, 36, 11321–11327 CrossRef CAS.
- M. R. Allen, V. R. Barros, J. Broome, W. Cramer, R. Christ, J. A. Church, L. Clarke, Q. Dahe, P. Dasgupta and N. K. Dubash, IPCC fifth assessment synthesis report, 2014.
- IEA, World Energy Outlook 2017, 2017.
- C. Munnings and A. Krupnick, Comparing Policies to Reduce Methane Emissions in the Natural Gas Sector, 2017.
- J. P. Marshall, Energy Policy, 2016, 99, 288–298 CrossRef.
- A. B. Rao and E. S. Rubin, Ind. Eng. Chem. Res., 2006, 45, 2421–2429 CrossRef CAS.
- A. J. Hunt, E. H. Sin, R. Marriott and J. H. Clark, ChemSusChem, 2010, 3, 306–322 CrossRef CAS PubMed.
- T. S. Chung, D. Patiño-Echeverri and T. L. Johnson, Energy Policy, 2011, 39, 5609–5620 CrossRef CAS.
- R. Bell, The Production of Low Carbon Gas–Consultation Response: SCCS response to the Carbon Connect consultation on the production of low carbon gas, 2018 Search PubMed.
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo and L. A. Hackett, Energy Environ. Sci., 2018, 11, 1062–1176 RSC.
- IEAGHG, Enabling the deployment of industrial CCS clusters, 2018.
- Global CCS Institute, The Global Status of CCS. Special Report: Understanding the Industrial CCS Hubs and Clusters, Melbourne, Australia, 2016.
- i24c, Deployment of an industrial carbon capture and storage cluster in Europe: A funding pathway, Cambridge, UK, 2017.
- I. Enting, D. Etheridge and M. J. Fielding, Int. J. Greenhouse Gas Control, 2008, 2, 289–296 CrossRef CAS.
- P. M. Haugan and F. Joos, Geophys. Res. Lett., 2004, 31, L18202 CrossRef.
- C. R. Jenkins, P. J. Cook, J. Ennis-King, J. Undershultz, C. Boreham, T. Dance, P. de Caritat, D. M. Etheridge, B. M. Freifeld and A. Hortle, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, E35–E41 CrossRef CAS PubMed.
- J. Banks and C. A. T. Force, Clean Air Task Force, 2012.
- U.S. EPA, Coal Mine Methane (CMM) Finance Guide, 2009.
- H. Yang, Z. Xu, M. Fan, R. Gupta, R. B. Slimane, A. E. Bland and I. Wright, J. Environ. Sci., 2008, 20, 14–27 CrossRef CAS.
- N. Muradov, Catal. Commun., 2001, 2, 89–94 CrossRef CAS.
- N. Muradov, Thermocatalytic CO2-free production of hydrogen from hydrocarbon fuels, 2000.
- G. Kreysa, ChemSusChem, 2009, 2, 49–55 CrossRef CAS PubMed.
- T. Keipi, H. Tolvanen and J. Konttinen, Energy Convers. Manage., 2018, 159, 264–273 CrossRef CAS.
- T. Abbasi and S. Abbasi, Renewable Sustainable Energy Rev., 2011, 15, 1828–1834 CrossRef CAS.
- M.-S. Liao and Q.-E. Zhang, J. Mol. Catal. A: Chem., 1998, 136, 185–194 CrossRef CAS.
- R. Aiello, J. E. Fiscus, H.-C. Zur Loye and M. D. Amiridis, Appl. Catal., A, 2000, 192, 227–234 CrossRef CAS.
- P. Tang, Q. Zhu, Z. Wu and D. Ma, Energy Environ. Sci., 2014, 7, 2580–2591 RSC.
- T. Geißler, A. Abánades, A. Heinzel, K. Mehravaran, G. Müller, R. Rathnam, C. Rubbia, D. Salmieri, L. Stoppel and S. Stückrad, Chem. Eng. J., 2016, 299, 192–200 CrossRef.
- M. D. Eisaman, J. L. B. Rivest, S. D. Karnitz, C.-F. de Lannoy, A. Jose, R. W. DeVaul and K. Hannun, Int. J. Greenhouse Gas Control, 2018, 70, 254–261 CrossRef CAS.
- R. Socolow, M. Desmond, R. Aines, J. Blackstock, O. Bolland, T. Kaarsberg, N. Lewis, M. Mazzotti, A. Pfeffer and K. Sawyer, Direct air capture of CO2 with chemicals: a technology assessment for the APS Panel on Public Affairs, American Physical Society, 2011 Search PubMed.
- World Energy Council, World Energy Resources, World Energy Council, London, UK, 2016.
- UK Government, International Industrial Energy Prices, (https://www.gov.uk/government/statistical-data-sets/international-industrial-energy-prices).
- European Environment Agency, Electricity Generation – CO2 emissions intensity, (https://www.eea.europa.eu/data-and-maps/daviz/co2-emission-intensity-3#tab-googlechartid_chart_11_filters=%7B“rowFilters”%3A%7B%7D%3B“columnFilters”%3A%7B“pre_config_ugeo”%3A%5B“European%20Union%20(28%20countries)”%5D%7D%7D, 2018).
- E. Lantz, M. Hand and R. Wiser, Past and Future Cost of Wind Energy: Preprint, National Renewable Energy Laboratory (NREL), Golden, CO., 2012 Search PubMed.
- S. Almosni, A. Delamarre, Z. Jehl, D. Suchet, L. Cojocaru, M. Giteau, B. Behaghel, A. Julian, C. Ibrahim and L. Tatry, Sci. Technol. Adv. Mater., 2018, 19, 336–369 CrossRef PubMed.
- M. R. Shaner, H. A. Atwater, N. S. Lewis and E. W. McFarland, Energy Environ. Sci., 2016, 9, 2354–2371 RSC.
- IRENA, Renewable Power Generation Costs in 2017, International Renewable Energy Agency, Abu Dhabi, 2018.
- G. F. Hewitt and J. G. Collier, Introduction to nuclear power, CRC Press, 2000 Search PubMed.
- N. Sakaba, H. Sato, H. Ohashi, T. Nishihara and K. Kunitomi, J. Nucl. Sci. Technol., 2008, 45, 962–969 CrossRef CAS.
- J. Norman, G. Besenbruch, L. Brown, D. O'keefe and C. Allen, Thermochemical water-splitting cycle, bench-scale investigations, and process engineering. Final report, February 1977-December 31, 1981, GA Technologies, Inc., San Diego, CA (USA), 1982.
- L. Brecher, S. Spewock and C. Warde, Int. J. Hydrogen Energy, 1977, 2, 7–15 CrossRef.
- N. E. Hultman, J. G. Koomey and D. M. Kammen, Environ. Sci. Technol., 2007, 2087–2094 CrossRef.
- J. R. Lovering, A. Yip and T. Nordhaus, Energy Policy, 2016, 91, 371–382 CrossRef.
- P. E. Dodds and W. McDowall, UCL Energy Institute, University College London, 2012.
- U.S. DOE, Book US Billion-Ton Update: Biomass Supply for a Bioenergy and Bioproducts Industry, 2011.
- IEAGHG, CO2Capture at coal based power and hydrogen plants, Report 2014/3, Cheltenham, UK, 2014.
- S. Fuss, J. G. Canadell, G. P. Peters, M. Tavoni, R. M. Andrew, P. Ciais, R. B. Jackson, C. D. Jones, F. Kraxner, N. Nakicenovic, C. Le Quere, M. R. Raupach, A. Sharifi, P. Smith and Y. Yamagata, Nat. Clim. Change, 2014, 4, 850–853 CrossRef CAS.
- V. S. Sikarwar, M. Zhao, P. Clough, J. Yao, X. Zhong, M. Z. Memon, N. Shah, E. J. Anthony and P. S. Fennell, Energy Environ. Sci., 2016, 9, 2939–2977 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ee02079e |
| ‡ 5th, 50th, 95th percentile and mean emissions are 0.3%, 0.9%, 3.3% and 1.6% respectively. Interquartile ranges of 0.6–1.4% have been used to refine the data set. These are results from a set of supply chain routes considered to be using modern and effective equipment. Note that this is different to the range of mean emissions (1.6–5.5%) seen across all supply chains mentioned in the previous paragraph. |
| This journal is © The Royal Society of Chemistry 2019 |