Proton-assisted air oxidation mechanisms of iron(ii) bis-thiosemicarbazone complexes at physiological pH: a kinetico-mechanistic study†
Abstract
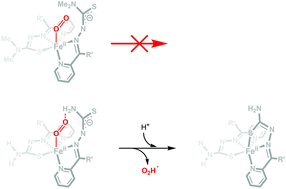
The kinetics of oxidation of different biologically-active FeII bis-thiosemicarbazone complexes in water has been monitored at varying dioxygen concentration, temperature, pressure, and pH. The oxidation reactions observed can be resolved as a single-step process, producing the expected ferric complex, with rates increasing with decreasing pH. From the pH-dependence of the observed rate constants, a rate law with two terms can be derived, one of them being independent of the acid concentration and the other term showing a saturation behaviour with respect to [H+]. These results indicate the existence of two parallel pathways for oxidation: the acid-independent pathway is only operative for the complexes with ligands bearing terminal, non-coordinated, unsubstituted amines, whereas the term with a [H+]-limiting kinetic behaviour is observed for all the complexes and indicates that the reacting species has to be protonated prior to the oxidation step. From the data collected, the rate law and the thermal and pressure activation parameters have been used to interpret the operating reaction mechanisms. Given the fact that the empirical trends rule out an outer-sphere oxidation process, DFT calculations have been carried out to explain the results and suggest the likely formation, under steady-state very low concentration conditions, of FeIII superoxo and hydroperoxo intermediates.
- This article is part of the themed collection: Inorganic Reaction Mechanisms


 Please wait while we load your content...
Please wait while we load your content...