Base-controlled mechanistic divergence between iron(iv)-oxo and iron(iii)-hydroperoxo in the H2O2 activation by a nonheme iron(ii) complex†
Abstract
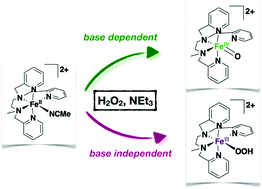
Activation of hydrogen peroxide by FeII salts (Fenton systems) leads to a myriad of oxidizing agents whose nature, FeIVO, or hydroxyl radicals and FeIII species, is dictated by the reaction conditions, in particular the pH value. Using the non heme FeII complex [FeII(L52)(CH3CN)]2+ (1) (where L52 is the pentadentate ligand N-methyl-N,N′,N′-tris(2-pyridylmethyl)ethane-1,2-diamine) we have observed the simultaneous formation of two reaction intermediates, [FeIV(O)(L52)]2+ and [FeIII(OOH)(L52)]2+, in its reaction with excess hydrogen peroxide in the presence of sub-stoichiometric amounts of triethylamine. Kinetic and spectroscopic monitoring of the reaction mixture and of independently prepared [FeIV(O)(L52)]2+ in the presence of the different constituents of the reaction mixture allows drawing a mechanistic scheme. These two reactive species are formed simultaneously following two independent and competitive pathways. [FeIV(O)(L52)]2+ is obtained via heterolytic O–O cleavage of the oxidant assisted by the base in a peroxidase-like mechanism whereas [FeIII(OOH)(L52)]2+ is generated upon homolytic O–O cleavage of hydrogen peroxide. The relative contribution of these two pathways can be tuned by adjusting the amount of base used.
- This article is part of the themed collection: Inorganic Reaction Mechanisms


 Please wait while we load your content...
Please wait while we load your content...