Acetylene and terminal alkyne complexes of copper(i) supported by fluorinated pyrazolates: syntheses, structures, and transformations†
Abstract
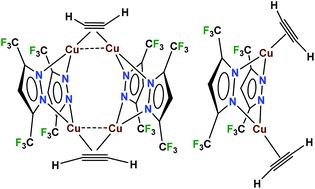
Trinuclear {μ-[3,5-(CF3)2Pz]Cu}3 reacts with acetylene to produce the 2 : 1 copper(I) acetylene complex, Cu4(μ-[3,5-(CF3)2Pz])4(μ-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH)2. Related Cu4(μ-[4-Br-3,5-(CF3)2Pz])4(μ-HC
CH)2. Related Cu4(μ-[4-Br-3,5-(CF3)2Pz])4(μ-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH)2 and Cu4(μ-[4-Cl-3,5-(CF3)2Pz])4(μ-HC
CH)2 and Cu4(μ-[4-Cl-3,5-(CF3)2Pz])4(μ-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH)2 have also been isolated using the corresponding copper(I) pyrazolate and acetylene. The 1 : 1 adducts Cu2(μ-[3,5-(CF3)2Pz])2(HC
CH)2 have also been isolated using the corresponding copper(I) pyrazolate and acetylene. The 1 : 1 adducts Cu2(μ-[3,5-(CF3)2Pz])2(HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH)2 and Cu2(μ-[4-Br-3,5-(CF3)2Pz])2(HC
CH)2 and Cu2(μ-[4-Br-3,5-(CF3)2Pz])2(HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH)2 are significantly less stable to the acetylene loss and can be observed in solution at low temperatures under excess acetylene. The X-ray crystal structures of 2 : 1 and 1 : 1 complexes, Cu4(μ-[3,5-(CF3)2Pz])4(μ-HC
CH)2 are significantly less stable to the acetylene loss and can be observed in solution at low temperatures under excess acetylene. The X-ray crystal structures of 2 : 1 and 1 : 1 complexes, Cu4(μ-[3,5-(CF3)2Pz])4(μ-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH)2 and Cu2(μ-[4-Br-3,5-(CF3)2Pz])2(HC
CH)2 and Cu2(μ-[4-Br-3,5-(CF3)2Pz])2(HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH)2 are reported. Raman data show a reduction in
CH)2 are reported. Raman data show a reduction in ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) C
C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C stretching frequency by about ∼340 and ∼163 cm−1 in the 2 : 1 and 1 : 1 Cu(I)/acetylene complexes, respectively, from that of the free acetylene. Copper(I) pyrazolate complexes of the terminal alkynes, phenylacetylene, 1,8-nonadiyne, and 1,7-octadiyne are also reported. They form adducts involving one copper atom on each alkyne moiety. The {μ-[3,5-(CF3)2Pz]Cu}3 is also a very versatile and competent catalyst for alkyne transformations as evident from its ability to catalyze the alkyne C(sp)–H bond carboxylation chemistry with CO2, azide–alkyne cycloadditions leading to 1,2,3-triazoles including the use of acetylene itself as a substrate, and thiol addition to phenylacetylene affording vinyl sulfides.
C stretching frequency by about ∼340 and ∼163 cm−1 in the 2 : 1 and 1 : 1 Cu(I)/acetylene complexes, respectively, from that of the free acetylene. Copper(I) pyrazolate complexes of the terminal alkynes, phenylacetylene, 1,8-nonadiyne, and 1,7-octadiyne are also reported. They form adducts involving one copper atom on each alkyne moiety. The {μ-[3,5-(CF3)2Pz]Cu}3 is also a very versatile and competent catalyst for alkyne transformations as evident from its ability to catalyze the alkyne C(sp)–H bond carboxylation chemistry with CO2, azide–alkyne cycloadditions leading to 1,2,3-triazoles including the use of acetylene itself as a substrate, and thiol addition to phenylacetylene affording vinyl sulfides.
- This article is part of the themed collections: Spotlight Collection: Fluorinated ligands and Philip Power at 65: an icon of organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...