A hybrid of g-C3N4 and porphyrin-based covalent organic frameworks via liquid-assisted grinding for enhanced visible-light-driven photoactivity†
Abstract
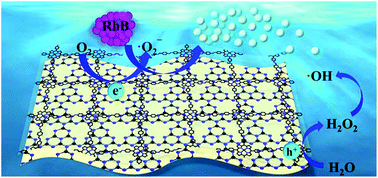
Designing photocatalysts with heterostructures is an effective way to promote visible-light-driven photocatalytic degradation. Herein, a series of 2D/2D heterojunction photocatalysts, denoted as CuPor-Ph-COF/g-C3N4 composites, were prepared through in situ synthesis on the surface of g-C3N4 by a facile liquid-assisted grinding method. The photocatalytic performance of the as-prepared CuPor-Ph-COF/g-C3N4 composites was evaluated by the degradation of a model pollutant rhodamine B. The CuPor-Ph-COF/g-C3N4 composites displayed superior photocatalytic performance to pure g-C3N4 or pure CuPor-Ph-COF because of the faster separation of photogenerated charges. This represents the first composite fabricated between a 2D porphyrin-based covalent organic framework (COF) and g-C3N4, demonstrating not only the possibility but also more importantly the affordability of the application of costly porphyrin-based COFs in catalysis.


 Please wait while we load your content...
Please wait while we load your content...