Relative stabilities of M/NHC complexes (M = Ni, Pd, Pt) against R–NHC, X–NHC and X–X couplings in M(0)/M(ii) and M(ii)/M(iv) catalytic cycles: a theoretical study†
Abstract
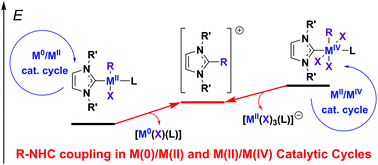
The complexes of Ni, Pd, and Pt with N-heterocyclic carbenes (NHCs) catalyze numerous organic reactions via proposed typical M0/MII catalytic cycles comprising intermediates with the metal center in (0) and (II) oxidation states. In addition, MII/MIV catalytic cycles have been proposed for a number of reactions. The catalytic intermediates in both cycles can suffer decomposition via R–NHC coupling and the side reductive elimination of the NHC ligand and R groups (R = alkyl, aryl, etc.) to give [NHC–R]+ cations. In this study, the relative stabilities of (NHC)MII(R)(X)L and (NHC)MIV(R)(X)3L intermediates (X = Cl, Br, I; L = NHC, pyridine) against R–NHC coupling and other decomposition pathways via reductive elimination reactions were evaluated theoretically. The study revealed that the R–NHC coupling represents the most favorable decomposition pathway for both types of intermediates (MII and MIV), while it is thermodynamically and kinetically more facile for the MIV complexes. The relative effects of the metal M (Ni, Pd, Pt) and ligands L and X on the R–NHC coupling for the MIV complexes were significantly stronger than that for the MII complexes. In particular, for the (NHC)2MIV(Ph)(Br)3 complexes, Ph–NHC coupling was facilitated dramatically from Pt (ΔG = −36.9 kcal mol−1, ΔG≠ = 37.5 kcal mol−1) to Pd (ΔG = −61.5 kcal mol−1, ΔG≠ = 18.3 kcal mol−1) and Ni (ΔG = −80.2 kcal mol−1, ΔG≠ = 4.7 kcal mol−1). For the MII oxidation state of the metal, the bis-NHC complexes (L = NHC) were slightly more kinetically and thermodynamically stable against R–NHC coupling than the mono-NHC complexes (L = pyridine). An inverse relation was observed for the MIV oxidation state of the metal as the (NHC)2MIV(R)(X)3 complexes were kinetically (4.3–15.9 kcal mol−1) and thermodynamically (8.0–23.2 kcal mol−1) significantly less stable than the (NHC)MIV(R)(X)3L (L = pyridine) complexes. For the NiIV and PdIV complexes, additional decomposition pathways via the reductive elimination of the NHC and X ligands to give the [NHC–X]+ cation (X–NHC coupling) or reductive elimination of the X–X molecule were found to be thermodynamically and kinetically probable. Overall, the obtained results demonstrate significant instability of regular Ni/NHC and Pd/NHC complexes (for example, not additionally stabilized by chelation) and high probability to initiate “NHC-free” catalysis in the reactions comprising MIV intermediates.


 Please wait while we load your content...
Please wait while we load your content...