Synthesis of a 3D free standing crystalline NiSex matrix for electrochemical energy storage applications†
Abstract
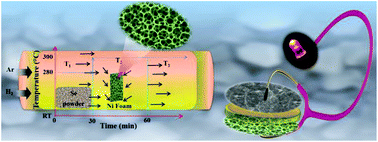
The electrochemical performance for energy storage of three-dimensional (3D) self-supported heterogeneous NiSex cubic-orthorhombic nanocrystals grown by a facile one-step chemical vapour deposition (CVD) approach on Ni foam substrates has been explored. NiSex shows a high specific capacitance of 1333 F g−1 with ultra-high energy (105 W h kg−1) and power (54 kW kg−1) densities. Furthermore, by integrating the as-grown NiSex as the anode and reduced graphene oxide as the cathode, a hybrid supercapacitor (HSC) prototype with a coin cell configuration has been fabricated. The device shows better capacitance (40 F g−1) with high energy (22 W h kg−1) and power (5.8 kW kg−1) densities and robust cycling durability (∼88% capacitance retention after 10 000 repeated cycles). For practical reliability of the as-fabricated HSC, a red LED has been illuminated by connecting it with two charged coin cells. These outstanding performances of the HSC prove to be promising for applications in high energy storage systems.


 Please wait while we load your content...
Please wait while we load your content...