Hydrogen peroxide adducts of triarylphosphine oxides†
Abstract
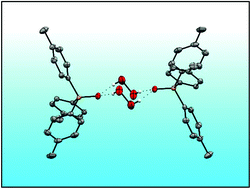
Five new hydrogen peroxide adducts of phosphine oxides (p-Tol3PO·H2O2)2 (1), (o-Tol3PO·H2O2)2 (2), (o-Tol2PhPO·H2O2)2 (3), (p-Tol3PO)2·H2O2 (4), and (o-TolPh2PO)2·H2O2 (5), and the water adduct (o-Tol2PhPO·H2O)2 (6) have been synthesized and fully characterized. Their single crystal X-ray structures have been determined and analyzed. The IR and 31P NMR data are in accordance with strong hydrogen bonding of the hydrogen peroxide. The mono- versus dimeric nature of the adduct assemblies has been investigated by DOSY NMR experiments. Raman spectroscopy of the symmetric adducts and the ν(O–O) stretching bands confirm the presence of hydrogen-bonded hydrogen peroxide in the solid materials. The solubilities in organic solvents have been quantified. Due to the high solubilities of 1–6 in organic solvents their 17O NMR spectra could be recorded in natural abundance, providing well-resolved signals for the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–O groups. The adducts 1–5 have been probed regarding their stability in solution at 105 °C. The decomposition of the adduct 1 takes place by loss of the active oxygen atoms in two steps.
O and O–O groups. The adducts 1–5 have been probed regarding their stability in solution at 105 °C. The decomposition of the adduct 1 takes place by loss of the active oxygen atoms in two steps.


 Please wait while we load your content...
Please wait while we load your content...
