A new tilt and an old twist on the nickel arsenide structure-type: synthesis and characterisation of the quaternary transition-metal cyanamides A2MnSn2(NCN)6 (A = Li and Na)†
Abstract
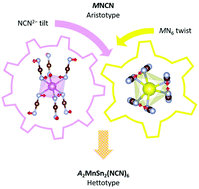
In this work, we describe the synthesis and structure of the quaternary transition-metal cyanamides Na2MnSn2(NCN)6 and Li2MnSn2(NCN)6. These phases crystallise isotypically in layered structures, with P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) 1m symmetry, that comprise hexagonal close-packed arrays of NCN2− anions with metal cations in 5/6 of the octahedral holes, thereby reflecting low-symmetry modifications of the hierarchical [NiAs]-type MNCN structure. The distinct coordination requirements of the metal cations template an ordered decoration across the octahedral sites with corundum-like [Sn2(NCN)3]2+ layers alternating with [A2Mn(NCN)3]2− layers which resemble a portion of the Li2Zr(NCN)3 structure. This motif is also mirrored in the form of the NCN2− anions which adopt N–C
1m symmetry, that comprise hexagonal close-packed arrays of NCN2− anions with metal cations in 5/6 of the octahedral holes, thereby reflecting low-symmetry modifications of the hierarchical [NiAs]-type MNCN structure. The distinct coordination requirements of the metal cations template an ordered decoration across the octahedral sites with corundum-like [Sn2(NCN)3]2+ layers alternating with [A2Mn(NCN)3]2− layers which resemble a portion of the Li2Zr(NCN)3 structure. This motif is also mirrored in the form of the NCN2− anions which adopt N–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N2− cyanamide shapes with clear single- and triple-bond character. Distortion-mode analysis reveals the importance of K1 octahedral twist and K2 cyanamide tilt displacements in stabilising these phases, the latter of which is only accessible because of the extended nature of the NCN2− anion. These are the first examples of non-binary transition-metal cyanamides to be discovered and this study highlights how the additional flexibility of the NCN2− anion affords a novel structure-type not observed in oxide chemistry.
N2− cyanamide shapes with clear single- and triple-bond character. Distortion-mode analysis reveals the importance of K1 octahedral twist and K2 cyanamide tilt displacements in stabilising these phases, the latter of which is only accessible because of the extended nature of the NCN2− anion. These are the first examples of non-binary transition-metal cyanamides to be discovered and this study highlights how the additional flexibility of the NCN2− anion affords a novel structure-type not observed in oxide chemistry.


 Please wait while we load your content...
Please wait while we load your content...