Water-mediated proton conduction in Ni(ii) and Co(ii) benzenetriphosphonates†
Abstract
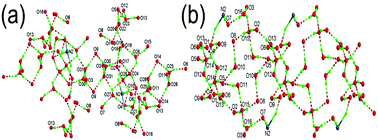
Two novel transition metal compounds, [Ni(4,4′-bipyH)2(H2O)4]·2(H4bmt)·9H2O (1) and [Co(4,4′-bipy)(H2O)4][Co(4,4′-bipyH)2(H2O)4]·2(H3bmt)·6H2O (2), have been synthesized. They possess continuous H-bond networks and high-density [PO3] groups, which give conductivity values in the order of 10−3 S cm−1 in a wide temperature range and 98% relative humidity.


 Please wait while we load your content...
Please wait while we load your content...