Synthesis of an MOF-based Hg2+-fluorescent probe via stepwise post-synthetic modification in a single-crystal-to-single-crystal fashion and its application in bioimaging†
Abstract
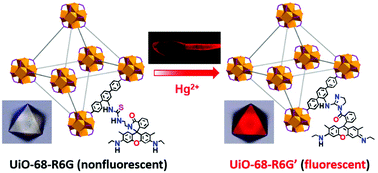
Although post-synthetic modification (PSM) has been successfully applied to NMOF decoration, only a handful of PSM-based single-crystal-to-single-crystal (SCSC) examples have been reported, particularly those involving multistep MOF-based SCSC transformations. In this contribution, three new MOFs, namely, UiO-68-NCS, UiO-68-R6G and UiO-68-R6G′, were prepared via the single-crystal-to-single-crystal post-synthetic modification approach. For bioimaging, nanosized UiO-68-NCS, UiO-68-R6G, and UiO-68-R6G′ were also prepared. Herein, nanosized UiO-68-R6G with a rhodamine-based fluorescence switch was found to be a highly sensitive and selective fluorescent probe for the detection of Hg2+ both in vitro and in vivo.


 Please wait while we load your content...
Please wait while we load your content...